Hydrothermal smectite synthesis in presence of Glycine: Synergistic chemical evolution
IF 5.8
2区 地球科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The role of smectite surfaces in organic matter dynamics is well documented, with clear evidence of their capacity to concentrate, preserve, and chemically modify organic compounds. However, the reciprocal influence—how organic molecules might guide clay mineral formation—remains a relatively unexplored scientific frontier. The present study investigates the dual role of glycine in Fe-smectite crystallization under hydrothermal conditions (150 °C for 5 days in an inert atmosphere), exploring how this ubiquitous amino acid influences mineral formation processes. X-ray diffraction confirmed the crystallization of Fe-smectite across glycine concentrations ranging from 0.08 to 0.6 M. The increase in glycine concentration induced a decrease in tetrahedral Fe(III) content and promoted the reduction of octahedral Fe(III) to Fe(II), as revealed by X-ray diffraction, Fourier transform infrared spectroscopy, and electrochemical analyses, consequently modifying the measured cation exchange capacity. Glycine polymerization was markedly enhanced in experiments with Fe-smectite crystallization compared to experiments without Fe-smectite. The presence of Fe-smectite favored linear peptides over cyclic one, thus facilitating the production of longer peptide chains. The results reveal a mutual interaction between glycine and minerals, resulting in minerals with distinct structural properties and reactivities that enhance organic matter transformation.
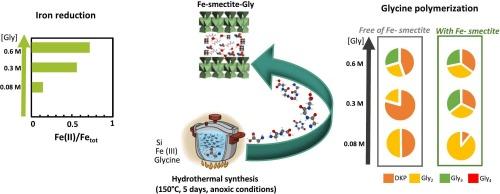
甘氨酸存在下水热合成蒙脱石:协同化学演化
蒙脱石表面在有机物质动力学中的作用是有据可证的,有明确的证据表明它们具有浓缩、保存和化学修饰有机化合物的能力。然而,相互影响——有机分子如何引导粘土矿物的形成——仍然是一个相对未被探索的科学前沿。本研究研究了甘氨酸在水热条件下(150°C惰性气氛中5天)铁蒙脱石结晶中的双重作用,探索了这种普遍存在的氨基酸如何影响矿物形成过程。x射线衍射证实了在0.08 ~ 0.6 m的甘氨酸浓度范围内铁蒙脱石的结晶。x射线衍射、傅里叶变换红外光谱和电化学分析表明,甘氨酸浓度的增加导致四面体铁(III)含量的降低,并促进八面体铁(III)还原为铁(II),从而改变了阳离子交换容量。与不含铁蒙脱石的实验相比,含铁蒙脱石的实验显著增强了甘氨酸的聚合。铁蒙脱石的存在有利于线性肽而不是环状肽,从而促进了长肽链的产生。结果表明,甘氨酸与矿物质之间存在相互作用,导致具有不同结构性质和反应活性的矿物质促进有机质转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Clay Science
地学-矿物学
CiteScore
10.30
自引率
10.70%
发文量
289
审稿时长
39 days
期刊介绍:
Applied Clay Science aims to be an international journal attracting high quality scientific papers on clays and clay minerals, including research papers, reviews, and technical notes. The journal covers typical subjects of Fundamental and Applied Clay Science such as:
• Synthesis and purification
• Structural, crystallographic and mineralogical properties of clays and clay minerals
• Thermal properties of clays and clay minerals
• Physico-chemical properties including i) surface and interface properties; ii) thermodynamic properties; iii) mechanical properties
• Interaction with water, with polar and apolar molecules
• Colloidal properties and rheology
• Adsorption, Intercalation, Ionic exchange
• Genesis and deposits of clay minerals
• Geology and geochemistry of clays
• Modification of clays and clay minerals properties by thermal and physical treatments
• Modification by chemical treatments with organic and inorganic molecules(organoclays, pillared clays)
• Modification by biological microorganisms. etc...
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


