Myelin–axon interface vulnerability in Alzheimer’s disease revealed by subcellular proteomics and imaging of human and mouse brain
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
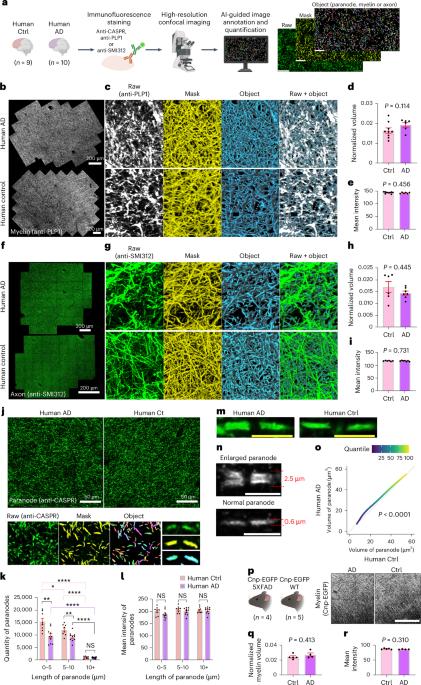
Myelin ensheathment is essential for rapid axonal conduction, metabolic support and neuronal plasticity. In Alzheimer’s disease (AD), disruptions in myelin and axonal structures occur, although the underlying mechanisms remain unclear. We implemented proximity labeling subcellular proteomics of the myelin–axon interface in postmortem human brains from AD donors and 15-month-old male and female 5XFAD mice. We uncovered multiple dysregulated signaling pathways and ligand–receptor interactions, including those linked to amyloid-β processing, axonal outgrowth and lipid metabolism. Expansion microscopy confirmed the subcellular localization of top proteomic hits and revealed amyloid-β aggregation within the internodal periaxonal space and paranodal/juxtaparanodal channels. Although overall myelin coverage is preserved, we found reduced paranode density, aberrant myelination and altered paranode positioning around amyloid-plaque-associated dystrophic axons. These findings suggest that the myelin–axon interface is a critical site of protein aggregation and disrupted neuro-glial signaling in AD. In Alzheimer’s disease (AD), disruptions in myelin and axonal structures occur, although the underlying mechanisms remain unclear. Here the authors show that, at the myelin–axon interface, axon–glial signaling, paranodal architecture and amyloid-β aggregation are altered in AD, implicating myelin–axon disruption in disease progression.

亚细胞蛋白质组学和人和小鼠脑成像揭示阿尔茨海默病髓磷脂-轴突界面易感性
髓鞘鞘是快速轴突传导、代谢支持和神经元可塑性所必需的。在阿尔茨海默病(AD)中,髓鞘和轴突结构发生破坏,尽管潜在的机制尚不清楚。我们对来自AD供体和15月龄雄性和雌性5XFAD小鼠的死后人脑髓磷脂-轴突界面进行了近距离标记亚细胞蛋白质组学研究。我们发现了多种失调的信号通路和配体-受体相互作用,包括与淀粉样蛋白-β加工、轴突生长和脂质代谢相关的信号通路。扩增显微镜证实了顶部蛋白质组的亚细胞定位,并发现淀粉样蛋白-β聚集在结间轴周空间和副结/旁结通道内。尽管总体髓鞘覆盖被保留,但我们发现淀粉样斑块相关的营养不良轴突周围的旁阳极密度降低,髓鞘形成异常和旁阳极定位改变。这些发现表明,髓鞘-轴突界面是阿尔茨海默病中蛋白质聚集和神经胶质信号中断的关键部位。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


