Dysbiosis of the gut microbiome may contribute to the pathogenesis of oral lichen planus through Treg dysregulation
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Oral lichen planus (OLP) is a chronic inflammatory disorder with autoimmune features and malignant transformation risk, lacking a definitive treatment, with CD4+ T cells being pivotal in its pathogenesis. Dysbiosis, an imbalance in the microbiome, is linked to various autoimmune and inflammatory diseases, where CD4+ T cells play a significant role. Given these insights, the development of OLP might be influenced by dysbiosis. This study investigates the association between dysbiosis and CD4+ T cells in OLP. We collected stool and saliva samples from OLP patients, conducting 16S rRNA gene analysis and mass spectrometry, and assessed CD4+ T cell characteristics in lesions through multiplex immunofluorescence and single-cell RNA sequencing. Peripheral blood samples were subjected to flow cytometry and cell culture assays. Results showed extensive gut dysbiosis in OLP patients, notably a reduction in short-chain fatty acid (SCFA)-producing bacteria essential for regulatory T cell (Treg) differentiation. While various CD4+ T cell subsets, including Tregs, were present in tissues, these Tregs as unresponsive to specific antigens, showing reduced immunosuppressive molecule expression. The decline in SCFA-producing bacteria correlated with fewer activated Tregs in tissues and blood. These findings suggest that gut dysbiosis may contribute to OLP by impairing Treg regulation, influencing disease pathogenesis.
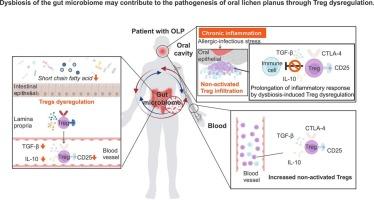
肠道菌群失调可能通过Treg失调导致口腔扁平苔藓的发病。
口腔扁平苔藓(OLP)是一种具有自身免疫特征和恶性转化风险的慢性炎症性疾病,缺乏明确的治疗方法,CD4+ T细胞在其发病机制中起关键作用。生态失调是一种微生物组失衡,与各种自身免疫性和炎症性疾病有关,其中CD4+ T细胞起着重要作用。鉴于这些见解,OLP的发展可能受到生态失调的影响。本研究探讨了OLP中生态失调与CD4+ T细胞的关系。我们收集OLP患者的粪便和唾液样本,进行16S rRNA基因分析和质谱分析,并通过多重免疫荧光和单细胞RNA测序评估病变中CD4+ T细胞的特征。外周血标本行流式细胞术和细胞培养检测。结果显示,OLP患者存在广泛的肠道生态失调,特别是产生短链脂肪酸(SCFA)的细菌减少,这是调节性T细胞(Treg)分化所必需的。虽然组织中存在各种CD4+ T细胞亚群,包括treg,但这些treg对特定抗原无反应,表现出免疫抑制分子表达减少。产生scfa的细菌的减少与组织和血液中活性treg的减少有关。这些发现表明,肠道生态失调可能通过损害Treg调节,影响疾病发病机制,从而促进OLP的发生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


