Protective exercise responses in the dentate gyrus of Alzheimer’s disease mouse model revealed with single-nucleus RNA-sequencing
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Exercise’s protective effects in Alzheimer’s disease (AD) are well recognized, but cell-specific contributions to this phenomenon remain unclear. Here we used single-nucleus RNA sequencing (snRNA-seq) to dissect the response to exercise (free-wheel running) in the neurogenic stem-cell niche of the hippocampal dentate gyrus in male APP/PS1 transgenic AD model mice. Transcriptomic responses to exercise were distinct between wild-type and AD mice, and most prominent in immature neurons. Exercise restored the transcriptional profiles of a proportion of AD-dysregulated genes in a cell type-specific manner. We identified a neurovascular-associated astrocyte subpopulation, the abundance of which was reduced in AD, whereas its gene expression signature was induced with exercise. Exercise also enhanced the gene expression profile of disease-associated microglia. Oligodendrocyte progenitor cells were the cell type with the highest proportion of dysregulated genes recovered by exercise. Last, we validated our key findings in a human AD snRNA-seq dataset. Together, these data present a comprehensive resource for understanding the molecular mediators of neuroprotection by exercise in AD. Dissecting the adaptive exercise response of the neurogenic stem-cell niche in the dentate gyrus by single-nucleus RNA sequencing reveals the molecular mediators that underlie exercise’s protective effects in Alzheimer’s disease.
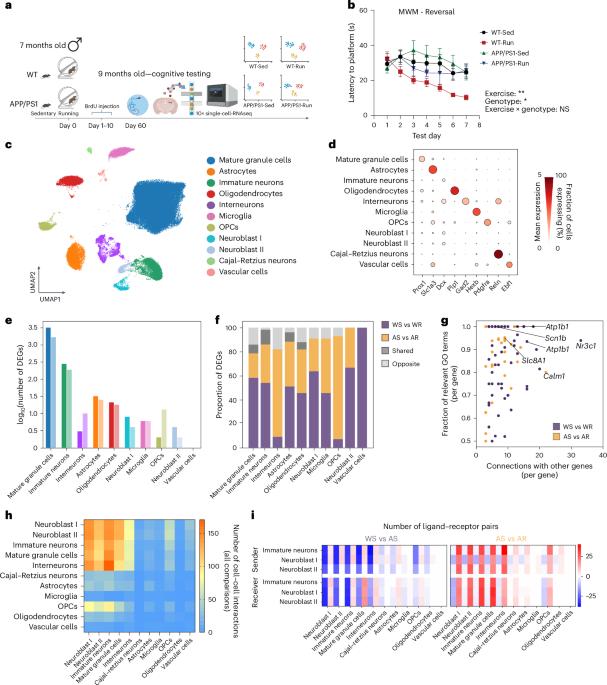

单核rna测序揭示阿尔茨海默病小鼠齿状回的保护性运动反应
运动对阿尔茨海默病(AD)的保护作用已得到充分认识,但细胞特异性对这一现象的贡献尚不清楚。本研究采用单核RNA测序技术(snRNA-seq)分析了APP/PS1转基因AD雄性模型小鼠海马齿状回神经源性干细胞生态位对运动(自由轮跑)的反应。运动的转录组反应在野生型和AD小鼠之间是不同的,并且在未成熟的神经元中最为突出。运动以细胞类型特异性的方式恢复了一部分ad失调基因的转录谱。我们发现了一个与神经血管相关的星形胶质细胞亚群,其丰度在阿尔茨海默病中降低,而其基因表达特征是通过运动诱导的。运动还增强了疾病相关小胶质细胞的基因表达谱。少突胶质祖细胞是运动恢复失调基因比例最高的细胞类型。最后,我们在人类AD snRNA-seq数据集中验证了我们的关键发现。总之,这些数据为理解运动对阿尔茨海默病患者神经保护的分子介质提供了全面的资源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


