Group 13 Lewis acid-mediated formation of 5-oxazolone derivatives from tert-butyl isocyanoacetate†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
tert-Butyl isocyanoacetate 1 reacted with B(C6F5)3 to give a Lewis acid–base adduct 2. GaCl3 and GaI3 promoted cyclization affording the N-bound Lewis acid adducts of the cyclized product 5-oxazolone derivatives 3 and 4, resulting from isocyano insertion into the ester C–O bond, with the loss of isobutylene. In contrast, the reactions with InBr3 and InI3 afforded the analogous adducts of 5(4H)-oxazolone derivatives 5 and 6, without the loss of the tert-butyl group. A proposed reaction mechanism is provided for these reactions of 1.
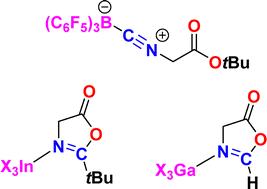
基团13:Lewis酸介导异氰乙酸叔丁酯生成5-恶唑酮衍生物
异氰乙酸叔丁酯1与B(C₆F₅)₃反应生成刘易斯酸碱加合物2。GaCl₃和GaI₃促进了环化,使环化产物5-恶唑酮衍生物3和4的n键路易斯酸加合物由异氰基插入酯C-O键而产生,同时损失了异丁烯。相反,5(4H)-恶唑酮衍生物5和6的类似加合物InBr₃和InI₃的形成没有损失叔丁基。提出了这些反应的反应机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


