Accessing carbon, boron and germanium spiro stereocentres in a unified catalytic enantioselective approach
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
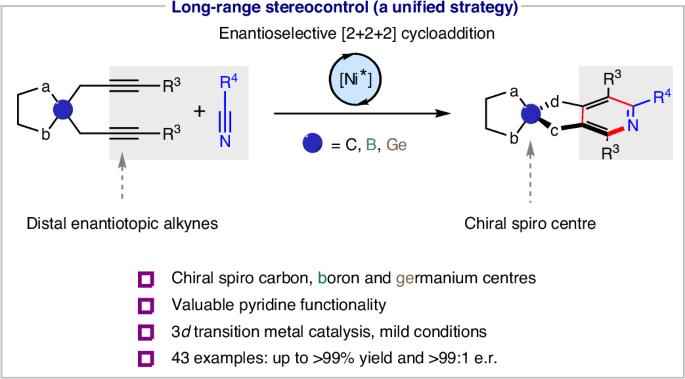
Achieving substrate generality in asymmetric catalysis remains a long-standing goal, particularly for the selective construction of chiral heteroatoms. Compared with carbon, sulfur, phosphorus and silicon stereogenic centres, methods for the construction of their boron and germanium congeners remain very scarce. Chiral (hetero) spirocycles are of relevance in several research domains. Methods effective for constructing carbon-centred chiral spirocycles do not translate to boron and germanium, leaving these chiral centres unexplored. We describe a unified strategy for constructing carbon, boron and germanium-centred chiral spirocyclic skeletons via enantioselective hetero [2+2+2] cycloaddition of a bis-alkyne with a nitrile. A chiral designer Ni(0) N-heterocyclic carbene complex enables the required long-range enantioinduction. The resulting enantio-enriched spirocycles feature a pyridine motif, making them exploitable for ligand design and functional materials featuring attractive photophysical and chiroptical properties. General methods to access stereogenic centres of different heteroatoms are limited. Now the nickel-catalysed enantioselective construction of carbon, boron and germanium-centred chiral spirocyclic frameworks via a hetero [2+2+2] cycloaddition of a bis-alkyne with a nitrile is presented.

在统一的催化对映选择方法中获得碳、硼和锗螺体中心
在不对称催化中实现底物的普遍性仍然是一个长期的目标,特别是对于手性杂原子的选择性构建。与碳、硫、磷和硅的立体中心相比,构建它们的硼和锗同系物的方法仍然很少。手性(异)螺环在许多研究领域都具有相关性。构建碳中心手性旋环的有效方法不能转化为硼和锗,使这些手性中心未被探索。我们描述了一种统一的策略,通过对映选择性异[2+2+2]环加成双炔和腈来构建以碳、硼和锗为中心的手性螺旋环骨架。手性设计物Ni(0) n -杂环羰基络合物实现了所需的远程对映体诱导。所得到的富含对映体的螺旋环具有吡啶基序,使其可用于配体设计和具有吸引的光物理和热学性质的功能材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


