Gold-Catalyzed Synthesis of Substituted 4-Pentafluorosulfanyl-1,3-Oxazoles
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
This work reports the first synthesis of pentafluorosulfanylated oxazoles through a gold(I)-catalyzed cyclization of SF5-alkynes and nitriles. It describes 18 examples of 4-SF5-oxazoles with NMR yield ranging from 3% to 75%. While the isolation of the pure pentafluorosulfanylated oxazoles proves challenging, the fluorinated heterocycles can nonetheless be obtained in yields between 14% and 52%, opening the door for their use as a novel fluorine-containing scaffold.
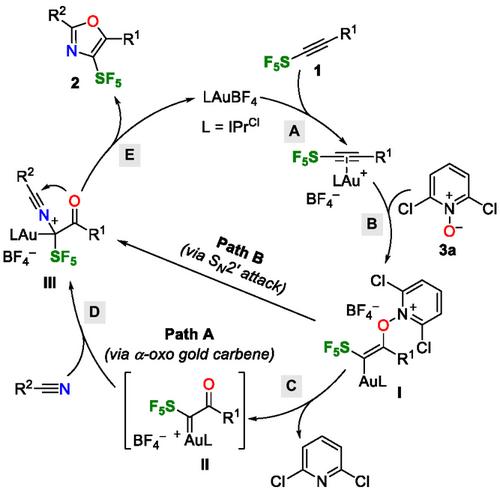
金催化取代4 -五氟磺酰- 1,3 -恶唑的合成
我们报道了首次通过金(I)催化SF5 -炔和腈的环化合成五氟磺酰化恶唑。我们描述了17个4‐SF5‐恶唑的例子,其核磁共振产率从3%到75%不等。虽然纯五氟磺酰化恶唑的分离被证明是具有挑战性的,但氟化杂环的产率仍在14%至52%之间,这为它们作为一种新型含氟支架的使用打开了大门。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


