Self-assembled ferritin nanoparticles using SpyCatcher/SpyTag multimerization of Mycobacterium tuberculosis TB10.4 protein induce potent immunogenicity
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Although Bacillus Calmette–Guérin (BCG) continues to play a role in alleviating tuberculosis as a global public health crisis, its potential to cause disseminated disease in immunocompromised individuals further limits its widespread use. However, traditional subunit vaccines face the challenge of weak immunogenicity. In this study, we employed the SpyCatcher/SpyTag system and a ferritin (Fer) nanoparticle (NP) to construct an NP-based vaccine targeting the non-region of difference antigen TB10.4. The construct comprises two components: a SpyCatcher003–Fer vector and SpyTag003–TB10.4 antigen, expressed in prokaryotic and eukaryotic systems, respectively. These components were self-assembled into the tuberculosis nanovaccine candidate, SpyCatcher–Fer–TB10.4 (SFT), in vitro. Compared with the monomeric TB10.4, subcutaneous immunization with SFT—without an adjuvant—markedly enhanced cell proliferation, promoted T lymphocyte activation, and stimulated multiple TB-related cytokines, with responses comparable to or exceeding those induced by BCG. These findings suggest that SFT is superior to conventional recombinant proteins and holds promise for eliciting immunoprotective effects similar to those of BCG in the future.
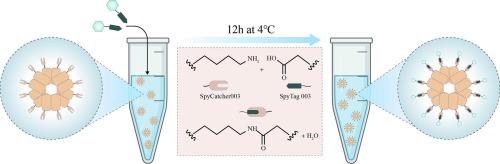
利用SpyCatcher/SpyTag聚合结核分枝杆菌TB10.4蛋白的自组装铁蛋白纳米颗粒诱导了强效免疫原性
虽然卡介苗继续在减轻结核病这一全球公共卫生危机方面发挥作用,但它可能在免疫功能低下的个体中引起播散性疾病,这进一步限制了它的广泛使用。然而,传统亚单位疫苗面临免疫原性弱的挑战。本研究采用SpyCatcher/SpyTag系统和铁蛋白(Fer)纳米颗粒(NP)构建了一种靶向非区差异抗原TB10.4的NP疫苗。该构建体由SpyCatcher003-Fer载体和SpyTag003-TB10.4抗原两部分组成,分别在原核和真核系统中表达。这些成分在体外自组装成结核纳米疫苗候选物SpyCatcher-Fer-TB10.4 (SFT)。与单体TB10.4相比,sft皮下免疫(不含佐剂)可显著增强细胞增殖,促进T淋巴细胞活化,并刺激多种结核病相关细胞因子,其反应可与BCG诱导的反应相当或超过BCG诱导的反应。这些发现表明,SFT优于传统的重组蛋白,并有望在未来引发类似卡介苗的免疫保护作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


