Intranasal delivery of platelet cell membrane-cloaked Astragaloside IV loaded biomimetic nanoparticles for enhanced therapeutics in acute lung injury mice
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-06-08
DOI:10.1016/j.ejpb.2025.114777
引用次数: 0
Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) frequently occur alongside sepsis, presenting significant challenges and associated mortality rates between 25 % and 40 %. Despite notable advancements in medical treatment, effective pharmacological options for ALI/ARDS remain limited due to rapid systemic clearance and insufficient targeting of lung tissues. To address this issue, we developed nanoparticles loaded with Astragaloside IV (ASIV-NPs) using an emulsification-evaporation method. Network pharmacology revealed 72 shared targets between ASIV and acute pneumonia, with core nodes (AKT1, CASP3, BCL2, IL6) identified through protein interaction analysis. Enrichment studies linked these targets to critical pathways including JAK-STAT signaling and cellular stress response. Molecular docking confirmed ASIV’s strong binding affinity (≤-5 kcal/mol) to key proteins governing inflammation and apoptosis. In vitro, PM@ASIV-NPs demonstrated biocompatibility, targeted inflamed macrophages, suppressed pro-inflammatory cytokines (IL-6, TNF-α, IL-1β), and scavenged ROS. In vivo imaging showed precise lung accumulation, while intranasal administration in ALI mice significantly enhanced IL-10, reduced inflammatory markers, and improved survival. The combined pharmacological evidence elucidates ASIV’s multimodal mechanism through target-pathway modulation, aligning with its observed therapeutic effects. This biomimetic nanoplatform utilizing platelet membrane camouflage offers a promising strategy for targeted ALI/ARDS treatment, with potential applicability to COVID-19-related pneumonia.
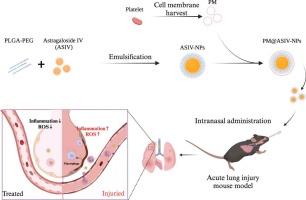
经鼻给药血小板细胞膜覆盖黄芪甲苷负载仿生纳米颗粒增强急性肺损伤小鼠的治疗效果
急性肺损伤(ALI)和急性呼吸窘迫综合征(ARDS)经常与败血症一起发生,带来重大挑战,相关死亡率在25%至40%之间。尽管医学治疗取得了显著进步,但由于急性呼吸窘迫综合征(ALI/ARDS)的快速全身清除和肺组织靶向性不足,有效的药物选择仍然有限。为了解决这个问题,我们使用乳化蒸发法开发了装载黄芪甲苷的纳米颗粒(ASIV-NPs)。网络药理学发现asv与急性肺炎共有72个靶点,通过蛋白相互作用分析鉴定出核心节点(AKT1、CASP3、BCL2、IL6)。富集研究将这些靶标与包括JAK-STAT信号和细胞应激反应在内的关键途径联系起来。分子对接证实了asv对炎症和细胞凋亡的关键蛋白具有很强的结合亲和力(≤-5 kcal/mol)。在体外,PM@ASIV-NPs显示出生物相容性,靶向炎症巨噬细胞,抑制促炎细胞因子(IL-6, TNF-α, IL-1β),并清除ROS。体内成像显示精确的肺积聚,而ALI小鼠鼻内给药可显著提高IL-10,减少炎症标志物,提高生存率。综合药理学证据阐明了ASIV通过靶标通路调节的多模态机制,与观察到的治疗效果一致。这种利用血小板膜伪装的仿生纳米平台为ALI/ARDS靶向治疗提供了一种有希望的策略,可能适用于covid -19相关肺炎。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


