Uranium uptake by phosphate minerals from Ca-containing technogenic solutions: Experimental study
引用次数: 0
Abstract
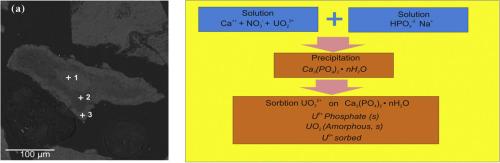
Since apatite is capable to adsorb and retain radionuclides, the removal of uranyl (UO22+) ions from two Ca-containing technogenic and model solutions (1–2 g/L of Ca) was studied when neutralized with sodium hydrogen phosphate solution Na2HPO4. Chemical composition of the sediments was analyzed by XPS and their structure was refined by XRD. The hydroxyapatite formation was confirmed. XRD proves that the two samples contained additional CaH(PO4) ∙ (H2O)2 (brushite), in which calcium changes to uranium of up to 15 % in a model solution. When identifying the oxidation state of uranium the presence of U4+, U5+ and U6+ was noticed with U5+ of up to 30–35 at. % from the total.
SEM-EDS did not allow to determine location of the phases with an uranium content over 14 %, for example Ca(UO2)2(PO4)2·11H2O (autunite) or other uranium phosphates. Uranium is detected at the grain rims, indicating a sorption nature of its accumulation. At the same time, thermodynamic computations showed the possible formation of independent uranium phases, such as ß-UO2.333, ß-UO2(OH)2 and NaUO2O(OH) (clarkeite), at the measured Eh-pH. We believe that the supersaturation of solutions and the spontaneous formation of hydroxyapatite and brushite solid particles led to a change in the initial Ca/PO4 ratio, where the P/Ca and Ca/O ratios, as well as the elemental composition in the near-surface layer of three sediments, are not strictly constant. There have been no similar studies on uranium, and our results demonstrate the need for further studies on the influence of uranium on apatite crystallization. Stability of phosphate phases shows the high efficiency of phosphate safety barriers for the uranium recovery facilities and these processes can be used for the water purification of polluted aquifers.
含钙工艺溶液中磷矿物对铀的吸收:实验研究
由于磷灰石能够吸附和保留放射性核素,研究了用磷酸氢钠溶液Na2HPO4中和两种含钙技术溶液和模型溶液(1 - 2g /L Ca)去除铀酰(UO22+)离子的情况。用XPS分析了沉积物的化学成分,并用XRD分析了沉积物的结构。证实了羟基磷灰石的形成。XRD结果表明,两种样品均含有CaH(PO4)∙(H2O)2(刷石),其中钙在模型溶液中转化为铀的比例高达15%。在鉴定铀的氧化态时,注意到U4+、U5+和U6+的存在,其中U5+高达30-35 at。从总额中抽取%。SEM-EDS无法确定铀含量超过14%的相的位置,例如Ca(UO2)2(PO4)2·11H2O(铀矿)或其他磷酸铀。在颗粒边缘检测到铀,表明其积累具有吸附性质。同时,热力学计算表明,在测量的Eh-pH下,可能形成独立的铀相,如ß-UO2.333、ß-UO2(OH)2和NaUO2O(OH) (clarkeite)。我们认为,溶液的过饱和和羟基磷灰石和刷石固体颗粒的自发形成导致了初始Ca/PO4比的变化,其中三种沉积物近表层的P/Ca和Ca/O比以及元素组成不是严格恒定的。目前还没有对铀的类似研究,我们的结果表明需要进一步研究铀对磷灰石结晶的影响。磷酸盐相的稳定性表明了铀回收设施中磷酸盐安全屏障的高效率,这些工艺可用于污染含水层的水净化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


