Fullerene Promotes CO2 Reduction to Methanol by a Cobalt(II) Phthalocyanine Electrocatalyst
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Heterogenization of molecular electrocatalysts offers an attractive way to improve the catalytic selectivity and efficiency of CO2 conversion to liquid fuels. Herein, we employ density functional theory to compare the mechanism of CO2RR by a cobalt(II) tetra(amino)phthalocyanine (Co(II)Pc(NH2)4) electrocatalyst with and without the presence of fullerene support. Our DFT calculations suggest that the CO2 reduction mechanism is initiated by a metal-based electron reduction followed by subsequent CO2 nucleophilic addition, electron transfer, proton transfer, water dissociation, and proton-coupled electron transfer steps that lead to CO and methanol formation. We show that graphitic interactions between the Co(II)Pc(NH2)4 electrocatalyst and C60 support selectively improve the CO2RR to methanol at mild potentials. The undesirable hydrogen evolution reaction (HER) was also investigated for both electrocatalysts and proceeds via the protonation of the cobalt metal center over the nitrogen atom in the inner ring. The competition between the HER and the CO2RR was improved in favor of CO and methanol formation using the Co(II)Pc(NH2)4@C60 electrocatalyst. Overall, our results suggest C60 as a promising graphitic support for molecular electrocatalysts integration for CO2 catalysis.
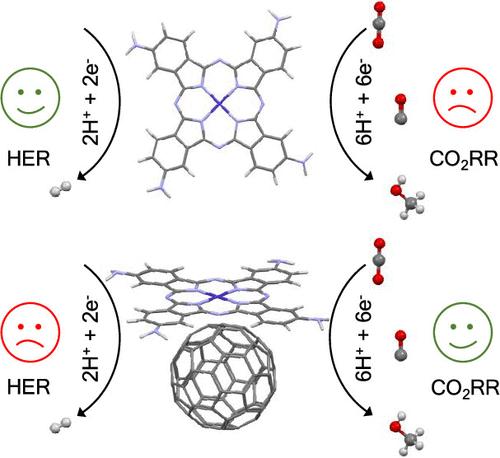
富勒烯通过钴(II)酞菁电催化剂促进CO2还原为甲醇
分子电催化剂的多相化为提高CO2转化为液体燃料的催化选择性和效率提供了一条有吸引力的途径。本文采用密度泛函理论比较了钴(II)四(氨基)酞菁(Co(II)Pc(NH2)4)电催化剂在富勒烯载体存在和不存在的情况下CO2RR的机理。我们的DFT计算表明,CO2还原机制是由金属基电子还原引发的,随后是CO2亲核加成、电子转移、质子转移、水解离和质子耦合电子转移步骤,导致CO和甲醇的形成。我们发现Co(II)Pc(NH2)4电催化剂与C60载体之间的石墨相互作用选择性地改善了CO2RR在温和电位下对甲醇的反应。研究了两种电催化剂的析氢反应,并通过钴金属中心在内环氮原子上的质子化进行了析氢反应。采用CO (II)Pc(NH2)4@C60电催化剂,改善了HER与CO2RR之间的竞争,有利于CO和甲醇的生成。综上所述,我们的研究结果表明C60是一种很有前途的用于二氧化碳催化的分子电催化剂集成的石墨载体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


