A flexible hippocampal population code for experience relative to reward
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
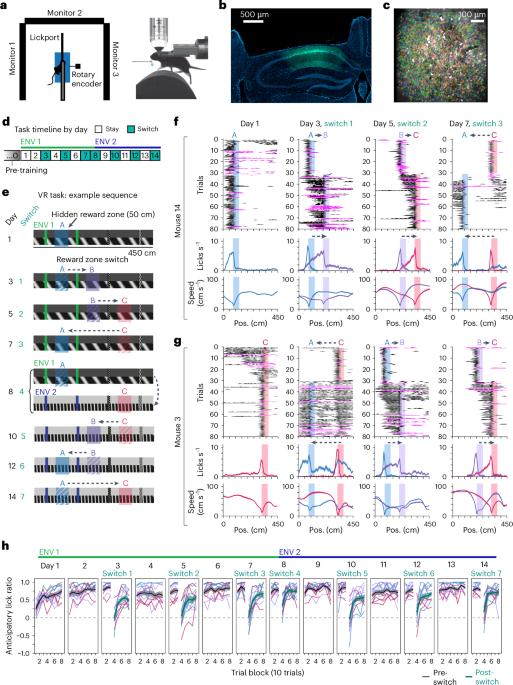
To reinforce rewarding behaviors, events leading up to and following rewards must be remembered. Hippocampal place cell activity spans spatial and non-spatial episodes, but whether hippocampal activity encodes entire sequences of events relative to reward is unknown. Here, to test this possibility, we performed two-photon imaging of hippocampal CA1 as mice navigated virtual environments with changing hidden reward locations. We found that when the reward moved, a subpopulation of neurons updated their firing fields to the same relative position with respect to reward, constructing behavioral timescale sequences spanning the entire task. Over learning, this reward-relative representation became more robust as additional neurons were recruited, and changes in reward-relative firing often preceded behavioral adaptations following reward relocation. Concurrently, the spatial environment code was maintained through a parallel, dynamic subpopulation rather than through dedicated cell classes. These findings reveal how hippocampal ensembles flexibly encode multiple aspects of experience while amplifying behaviorally relevant information. Sosa et al. find that hippocampal neural activity in mice encodes both environmental location and experience relative to rewards, spanning distances far from reward, through parallel and flexible population-level codes.

一个灵活的海马体群体编码相对于奖励的经验
为了强化奖励行为,必须记住奖励之前和之后的事件。海马体位置细胞活动跨越空间和非空间事件,但海马体活动是否编码与奖励相关的整个事件序列尚不清楚。在这里,为了测试这种可能性,我们对小鼠在具有变化的隐藏奖励位置的虚拟环境中导航时的海马CA1进行了双光子成像。我们发现,当奖励移动时,神经元亚群将它们的放电场更新到与奖励相同的相对位置,构建跨越整个任务的行为时间尺度序列。在学习过程中,随着额外的神经元被招募,这种奖励相关表征变得更加强大,奖励相关放电的变化通常先于奖励重新定位后的行为适应。同时,空间环境代码是通过一个并行的、动态的亚种群而不是通过专门的细胞类来维持的。这些发现揭示了海马体在放大行为相关信息的同时如何灵活地编码经验的多个方面。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


