Layered transition metal oxides with regulated phase transition and low interlayer spacing variation for sodium-ion batteries
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Ni/Mn-based layered transition metal oxides (LTMOs) without the involvement of expensive Co are regarded as promising cathode materials for sodium-ion batteries. Severe phase transitions at high charge voltages would result in significant variation in interlayer spacing, deteriorating cycling performance. Designing LTMOs with low interlayer spacing variation through the regulated phase transition remains a significant challenge. Herein, Na0.67Ni0.20Zn0.05Mn0.55Ti0.20O2 (NZMT) materials are synthesized through the co-substitution of Zn and Ti at the transition metal sites, Ni and Mn, respectively, of Na0.67Ni0.25Mn0.75O2 (NM). In situ X-ray diffraction analyses confirm that the resultant NZMT undergoes the regulated P2-OP4 phase transition, rather than the conventional P2-O2 phase transition or the multi-step phase transformation observed in NM. The interlayer spacing is marginally decreased from 5.6 Å in the P2 phase to 5.4 Å in the OP4 phase for NZMT. A discharge capacity of 99.9 mAh g−1 is delivered by NZMT at 1 C, with a capacity retention of 79.4 % after 200 cycles, significantly higher than those of NM (78.2 mAh g−1, 29.3 % retention). A high energy density of 274.3 Wh kg−1 is achieved for NZMT-based full cell at a power density of 78.3 W kg−1. This work paves a new avenue for the development of LTMOs.
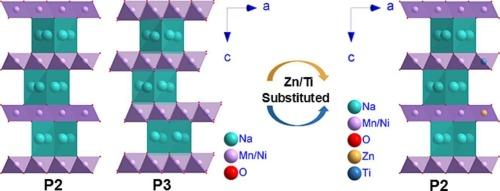
钠离子电池用具有可调节相变和低层间距变化的层状过渡金属氧化物
镍/锰基层状过渡金属氧化物(LTMOs)被认为是一种很有前途的钠离子电池正极材料。在高充电电压下,剧烈的相变会导致层间距的显著变化,使循环性能恶化。通过调节相变设计具有低层间距变化的LTMOs仍然是一个重大挑战。本文通过在Na0.67Ni0.25Mn0.75O2 (NM)的过渡金属位Ni和Mn上分别用Zn和Ti共取代,合成了na0.67 ni0.20 zn0.05 mn0.55 ti0.200 o2 (NZMT)材料。原位x射线衍射分析证实,所得的NZMT经历了调控的P2-OP4相变,而不是传统的P2-O2相变或NM中观察到的多步相变。NZMT的层间间距从P2阶段的5.6 Å略微减小到OP4阶段的5.4 Å。NZMT在1℃下的放电容量为99.9 mAh g−1,在200次 次循环后,容量保持率为79.4% %,显著高于NM (78.2 mAh g−1,29.3 %)。在78.3 W kg−1的功率密度下,获得了274.3 Wh kg−1的能量密度。这项工作为ltmo的发展开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


