Advanced 64Cu/67Cu-Based Theranostics: Application of the Copper Chelator TE1PA to a Murine Model of HER2-Positive Gastric Cancer
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The development of 64Cu/67Cu-based theranostic probes addresses the need for integrated cancer diagnosis and therapy. To target HER2-positive gastric cancer, trastuzumab was conjugated to p-SCN-Bn-TE1PA (DOL of 1.1–2.0), achieving high yields and purity. Binding assays on BT-474 cells confirmed the preserved cellular uptake of the bioconjugate. The successful radiolabeling of Trastuzumab-p-SCN-Bn-TE1PA (DOL of 2) with both the 64Cu- and 67Cu-isotopes demonstrated suitability for in vivo studies. In a preclinical model of HER2-positive gastric cancer, [64Cu]Cu-Trastuzumab-p-SCN-Bn-TE1PA enabled effective PET imaging with tumor-specific uptake (>21%IA/g) and clearance through the spleen, liver and kidneys. Therapeutic studies with [67Cu]Cu-Trastuzumab-p-SCN-BnTE1PA (10–20 MBq) demonstrated dose-dependent tumor inhibition of growth, without toxicity or adverse health effects. This work exemplifies the clinical potential of associating 64Cu-imaging and 67Cu-therapy. By combining a robust isotopic pair, a customized bifunctional chelator and trastuzumab, this study demonstrates a promising approach for HER2-positive gastric cancer treatment in nuclear medicine, paving the way for personalized copper-based theranostics.
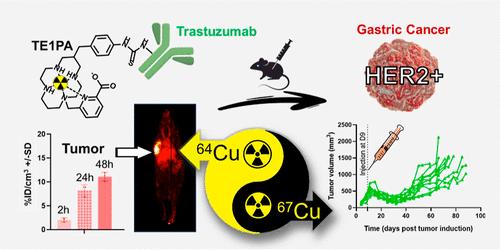
基于64Cu/ 67cu的先进治疗:铜螯合剂TE1PA在her2阳性胃癌小鼠模型中的应用
基于64Cu/ 67cu的治疗探针的开发满足了癌症综合诊断和治疗的需求。为了靶向her2阳性胃癌,曲妥珠单抗偶联p-SCN-Bn-TE1PA (DOL为1.1-2.0),获得了高产量和高纯度。BT-474细胞的结合实验证实了生物偶联物的细胞摄取。Trastuzumab-p-SCN-Bn-TE1PA (DOL为2)与64Cu-和67cu -同位素的成功放射性标记证明了其在体内研究中的适用性。在her2阳性胃癌的临床前模型中,[64Cu]Cu-Trastuzumab-p-SCN-Bn-TE1PA能够实现有效的PET成像,具有肿瘤特异性摄取(>21%IA/g),并通过脾脏、肝脏和肾脏清除。[67Cu] cu -曲妥珠单抗-p- scn - bnte1pa (10 - 20mbq)的治疗研究表明,肿瘤生长抑制具有剂量依赖性,无毒性或不良健康影响。这项工作证明了64cu成像与67cu治疗相关联的临床潜力。通过结合强大的同位素对、定制的双功能螯合剂和曲妥珠单抗,该研究展示了一种有希望的her2阳性胃癌治疗方法,为个性化铜基治疗铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


