Molecular Dynamics Simulations of a Putative Novel Mechanism for UCP1-Assisted FA Anion Transport
Abstract
Background
Mitochondrial energy can be stored as ATP or released as heat by uncoupling protein 1 (UCP1) during non-shivering thermogenesis in brown adipose tissue. UCP1, located in the inner mitochondrial membrane, reduces the proton gradient in the presence of long-chain fatty acids (FA). FA act as weak, protein-independent uncouplers, with the transport of the FA anion across the membrane being the rate-limiting step. According to the fatty acid cycling hypothesis, UCP1 catalyzes this step through an as-yet-undefined mechanism.
Methods
We used computational and experimental techniques, including all-atom molecular dynamics (MD) simulations, membrane conductance measurements, and site-directed mutagenesis.
Results
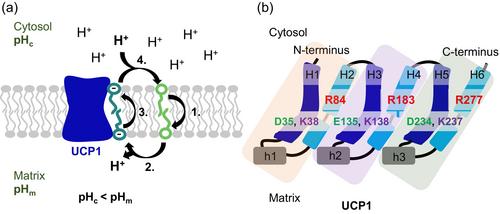
We identified two novel pathways for fatty acid anion translocation (sliding) at the UCP1 protein–lipid interface, ending at key arginine residues R84 and R183 in a nucleotide-binding region. This region forms a stable complex with fatty acid anion, which is crucial for anion transport. Mutations of these two arginines reduced membrane conductance, consistent with the MD simulation prediction that the arachidonic acid anion slides between helices H2–H3 and H4–H5, terminating at R84 and R183. Protonation of the arachidonic acid anion predicts its release from the protein–lipid interface, allowing it to move to either cytosolic or matrix leaflets of the membrane.
Conclusion
We provide a novel, detailed mechanism by which UCP1 facilitates fatty acid anion transport, as part of the fatty acid cycling process originally proposed by Skulachev. The residues involved in this transport are conserved in other SLC25 proteins, suggesting the mechanism may extend beyond UCP1 to other members of the superfamily.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


