Purification of astrazon pink FG and brilliant Red B contaminated water using zeolitic imidazolate framework enhanced potassium ferrite
IF 4.3
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
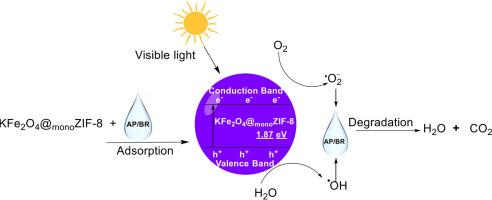
Adsorption and photocatalysis are known methods for removing dyes from aqueous systems. However, they may suffer from shortcomings like poor dye removal, high cost, poor adsorbent/catalyst regeneration and poor adsorbent/catalyst recovery. Therefore, a zeolitic imidazolate framework enhanced potassium ferrite (KFe2O4@monoZIF-8) was prepared to remove astrazon pink FG (AP) and brilliant Red B (BR) dyes from the water system. The characterization results of KFe2O4@monoZIF-8 revealed a well-structured diffraction pattern with a crystallite size of 31.24 nm and an energy bandgap of 1.87 eV. The scanning electron micrograph image revealed a homogeneous surface with similarly shaped particles of different sizes. At the same time, the energy-dispersive X-ray spectroscopy and elemental mapping confirmed the component elements to be K, Fe, O, C and Zn. Interestingly, KFe2O4@monoZIF-8 functions as an adsorbent and a photocatalyst. The adsorption of AP and BR by KFe2O4@monoZIF-8 in the absence of visible light revealed an equilibrium sorption capacity of 17.85±0.8 and 15.00±0.8 mg g-1, respectively, in a process described by pseudo-2nd-order kinetic model. Furthermore, when subjected to photocatalytic degradation under visible light irradiation, the removal efficiencies towards AP and BR became 98.80±1.20 and 95.2 ± 1.30 %, respectively. KFe2O4@monoZIF-8 in a binary mixed solution of AP and BR exhibited a photodegradation efficiency of 78.00±1.10 and 70.00±1.10 % towards AP and BR, respectively. In addition, KFe2O4@monoZIF-8 exhibited a regeneration capacity above 70 % at the 7th regeneration treatment cycle. KFe2O4@monoZIF-8 compared favourably with previously published materials for removing dyes in an aqueous solution. This study revealed KFe2O4@monoZIF-8 as a promising material for removing dyes from the water system.
沸石咪唑酸骨架强化铁酸钾净化黄芪粉FG和亮红B污染水
吸附和光催化是从水系统中去除染料的已知方法。但存在去除率差、成本高、吸附剂/催化剂再生能力差、吸附剂/催化剂回收率低等缺点。为此,制备了咪唑酸分子筛骨架增强铁酸钾(KFe2O4@monoZIF-8),以去除水中的黄褐红FG (AP)和亮红B (BR)染料。表征结果显示KFe2O4@monoZIF-8具有结构良好的衍射图样,晶粒尺寸为31.24 nm,能带隙为1.87 eV。扫描电镜图像显示了一个均匀的表面,具有形状相似的不同大小的颗粒。同时,能量色散x射线能谱和元素作图证实了组成元素为K、Fe、O、C和Zn。有趣的是,KFe2O4@monoZIF-8作为吸附剂和光催化剂。在无可见光条件下,KFe2O4@monoZIF-8吸附AP和BR的平衡吸附量分别为17.85±0.8 mg g-1和15.00±0.8 mg g-1,吸附过程符合拟二级动力学模型。在可见光照射下进行光催化降解,对AP和BR的去除率分别为98.80±1.20%和95.2±1.30%。KFe2O4@monoZIF-8在AP和BR二元混合溶液中对AP和BR的光降解效率分别为78.00±1.10%和70.00±1.10%。在第7个再生处理周期,KFe2O4@monoZIF-8的再生能力达到70%以上。KFe2O4@monoZIF-8与以前发表的用于去除水溶液中的染料的材料相比,效果更好。该研究表明KFe2O4@monoZIF-8是一种很有前途的去除水系统染料的材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Physics Impact
Materials Science-Materials Science (miscellaneous)
CiteScore
2.60
自引率
0.00%
发文量
65
审稿时长
46 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


