Enhanced reactive oxygen species mediated dye-degradation by H2O2 activation with different MoS2 nanostructures
IF 5.9
3区 工程技术
Q1 CHEMISTRY, MULTIDISCIPLINARY
Journal of Industrial and Engineering Chemistry
Pub Date : 2025-02-17
DOI:10.1016/j.jiec.2025.02.033
引用次数: 0
Abstract
Organic dyes, extensively utilised in industry, pose significant environmental risks in wastewater. Exploring the catalytic potential of different forms of molybdenum disulfide (MoS2) to address organic dye pollution is essential for environmental remediation. We demonstrate accelerated dye degradation assisted by an advanced oxidation process (AOP) in the dark, using progressively exfoliated forms of MoS2 (bulk, nanoflakes, and nanosheets) in the presence of hydrogen peroxide (H2O2). MoS2 nanosheets, exhibit ∼ 11 and 6 folds higher efficiency in the defect-mediated catalytic generation of hydroxyl (•OH) radical and singlet oxygen, respectively, than bulk, as revealed by terephthalic acid and 1,3 diphenylisobenzofuran assays, respectively. Direct electron paramagnetic resonance spectroscopy confirms at least 37 % higher generation of •OH radicals in the MoS2 nanosheets than in the bulk. The AOP-assisted chemical degradation efficiency of methylene blue (MB) is ∼ 28, ∼ 73, and ∼ 89 % for MoS2 bulk, nanoflakes, and nanosheets, respectively, over 1 hr. MoS2 nanosheets can also chemically degrade other organic dyes, such as celestine blue (∼80 %) and malachite green (∼48 %). The dye-degradation mechanism is elucidated via the pseudo-first-order kinetic curve of catalytic activity of different MoS2 forms with reaction constants of 0.00578, 0.02603, and 0.03463 min−1 for bulk, nanoflakes, and nanosheets, respectively.
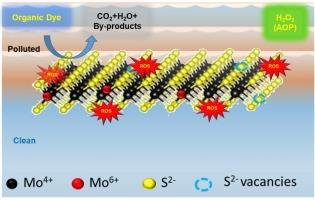
不同二硫化钼纳米结构活化H2O2增强活性氧介导的染料降解
有机染料在工业中广泛使用,其废水对环境造成了严重的危害。探索不同形态的二硫化钼(MoS2)对有机染料污染的催化潜力是环境修复的重要内容。我们展示了在黑暗中使用逐渐脱落的二硫化钼(大块、纳米片和纳米片)在过氧化氢(H2O2)存在下,由高级氧化过程(AOP)辅助的加速染料降解。对苯二甲酸和1,3二苯基异苯并呋喃实验显示,MoS2纳米片在缺陷介导的羟基(•OH)自由基和单线态氧的催化生成效率分别比块体高11倍和6倍。直接电子顺磁共振谱证实,在MoS2纳米片中,•OH自由基的生成比在本体中至少高出37%。aop辅助的亚甲基蓝(MB)在1小时内对二硫化钼体、纳米片和纳米片的化学降解效率分别为~ 28%、~ 73%和~ 89%。二硫化钼纳米片也可以化学降解其他有机染料,如天青石蓝(~ 80%)和孔雀石绿(~ 48%)。通过反应常数分别为0.00578、0.02603和0.03463 min−1的不同形式二硫化钼的催化活性拟一级动力学曲线,阐明了染料降解机理。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
6.60%
发文量
639
审稿时长
29 days
期刊介绍:
Journal of Industrial and Engineering Chemistry is published monthly in English by the Korean Society of Industrial and Engineering Chemistry. JIEC brings together multidisciplinary interests in one journal and is to disseminate information on all aspects of research and development in industrial and engineering chemistry. Contributions in the form of research articles, short communications, notes and reviews are considered for publication. The editors welcome original contributions that have not been and are not to be published elsewhere. Instruction to authors and a manuscript submissions form are printed at the end of each issue. Bulk reprints of individual articles can be ordered. This publication is partially supported by Korea Research Foundation and the Korean Federation of Science and Technology Societies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


