Enhancing antimicrobial efficacy: Gum acacia-enriched Lactobacillus consortium against multidrug-resistant pathogens
Q2 Medicine
引用次数: 0
Abstract
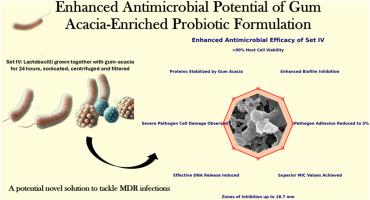
The escalating challenge of antimicrobial resistance (AMR) has rendered many pathogens impervious to conventional antibiotics, necessitating novel therapeutic approaches. This study evaluates the antimicrobial potential of a Gum Acacia-enriched Active GRAS Consortium (AGC), composed of Lactobacillus plantarum, L. acidophilus, and L. casei var. rhamnosus, against multidrug-resistant (MDR) pathogens. AGC formulations, with (Set IV) and without (Set III) gum acacia, were tested against clinical isolates of Staphylococcus aureus, Acinetobacter baumannii, Klebsiella pneumoniae, and Escherichia coli. Set IV demonstrated significantly enhanced antimicrobial activity, with inhibition zones reaching 18.7 mm, minimum inhibitory concentrations (MIC) as low as 0.83 mg/ml, and strong biofilm inhibition. Mechanistic assays revealed amplified DNA release from treated pathogens and unique antimicrobial protein profiles (<6.5 kDa) stabilized by gum acacia. Scanning electron microscopy confirmed severe ultrastructural damage, including membrane disruption and lysis, in Set IV-treated pathogens. Adhesion assays on Caco-2 cells highlighted Set IV's ability to reduce pathogen adhesion to 5 %, outperforming non-GA enriched formulation. These results underscore the synergistic role of probiotics and natural polysaccharides, like gum acacia, in combating MDR pathogens. Set IV's dual action-direct pathogen inhibition and biofilm disruption-positions it as a promising candidate for alternative or adjunctive therapies in AMR management. This study highlights the potential of integrating probiotics and prebiotics to create innovative solutions addressing the global AMR crisis.
增强抗菌功效:富含金合欢胶的乳酸杆菌联合体对抗多重耐药病原体
抗菌素耐药性(AMR)不断升级的挑战已经使许多病原体对传统抗生素不敏感,需要新的治疗方法。本研究评估了由植物乳杆菌、嗜酸乳杆菌和鼠李糖干酪乳杆菌组成的富含相思胶的活性GRAS联合体(AGC)对多药耐药(MDR)病原体的抗菌潜力。AGC制剂,含(组IV)和不含(组III)相思胶,对临床分离的金黄色葡萄球菌、鲍曼不动杆菌、肺炎克雷伯菌和大肠杆菌进行了检测。菌株IV的抑菌活性显著增强,抑菌区达18.7 mm,最低抑菌浓度(MIC)低至0.83 mg/ml,具有较强的生物膜抑制作用。机制分析显示,经处理的病原体释放的DNA扩增,以及由金合欢胶稳定的独特抗菌蛋白谱(<6.5 kDa)。扫描电子显微镜证实,在Set iv处理的病原体中存在严重的超微结构损伤,包括膜破坏和裂解。Caco-2细胞的粘附试验突出了Set IV将病原体粘附率降低至5%的能力,优于非ga富集制剂。这些结果强调了益生菌和天然多糖(如阿拉伯胶)在对抗耐多药病原体中的协同作用。Set IV的双重作用-直接抑制病原体和破坏生物膜-使其成为AMR管理中替代或辅助治疗的有希望的候选药物。这项研究强调了整合益生菌和益生元的潜力,以创造解决全球抗生素耐药性危机的创新解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Medicine in Microecology
Medicine-Gastroenterology
CiteScore
5.60
自引率
0.00%
发文量
16
审稿时长
76 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


