Interferon-epsilon, an estrogen-induced type I interferon, is uniquely exploited by Neisseria gonorrhoeae via effects on sialic acid metabolism
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
The female genital mucosa expresses the hormone-dependent type I interferon (IFN), IFN-epsilon (IFN-ε), which protects against chlamydia and herpes infection. Surprisingly, we found that IFN-ε knockout (Ifnε−/−) mice and type I IFN receptor knockout (Ifnar1−/−) mice exhibited enhanced clearance of Neisseria gonorrhoeae (Ng). This result was phenocopied using blocking anti-IFNAR monoclonal antibody (mAb). Ng colonization of the Ifnε−/− urogenital tract was restored by exogenous recombinant IFN-ε or IFN-β. Clearance of Ng in anti-IFNAR-treated mice required the expression of the cathelicidin mCRAMP. Ng deploys a unique mechanism to evade cathelicidins and other innate defenses by sialylating its lipooligosaccharide (LOS) using host-derived cytidine-5'-monophospho-N-acetylneuraminic acid (CMP-Neu5Ac or CMP-sialic acid). Ifnε−/− mice expressed reduced levels of CMP-sialic acid synthetase mRNA in genital tissues. Accordingly, Ng recovered from IFN-deficient mice were hyposialylated. In conclusion, Ng exploits type I IFNs to obtain CMP-sialic acid for LOS sialylation, resulting in innate immune evasion and enhanced colonization.
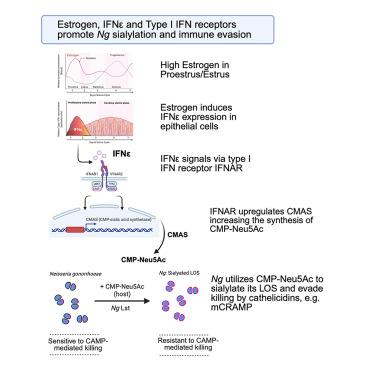
干扰素-epsilon是一种雌激素诱导的I型干扰素,是淋病奈瑟菌通过影响唾液酸代谢而独特利用的干扰素
女性生殖器粘膜表达激素依赖性I型干扰素(IFN), IFN-ε (IFN-ε),具有预防衣原体和疱疹感染的作用。令人惊讶的是,我们发现IFN-ε敲除(IFN ε−/−)小鼠和I型IFN受体敲除(Ifnar1−/−)小鼠对淋病奈瑟菌(Ng)的清除能力增强。该结果使用阻断抗ifnar单克隆抗体(mAb)进行表型复制。外源性重组IFN-ε或IFN-β可恢复Ng在泌尿生殖道的定殖。在抗ifnar处理的小鼠中清除Ng需要表达cathelicidin mCRAMP。Ng利用宿主衍生胞苷-5′-单磷酸- n -乙酰神经氨酸(CMP-Neu5Ac或cmp -唾液酸)唾液化其脂寡糖(LOS),利用独特的机制逃避抗菌素和其他先天防御。Ifnε−/−小鼠生殖器组织中cmp -唾液酸合成酶mRNA表达水平降低。因此,从缺乏ifn的小鼠中恢复的Ng是低磷酸化的。综上所述,Ng利用I型ifn获得cmp -唾液酸,用于LOS唾液酰化,导致先天免疫逃避和增强定植。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


