Rechallenge with immune-checkpoint inhibitors in patients with advanced-stage lung cancer
IF 82.2
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
Lung cancer remains the leading cause of cancer-related mortality globally, with many patients diagnosed with advanced-stage disease. Treatment in this setting relies on systemic therapies, including chemotherapy, targeted therapy and immunotherapy. Immune-checkpoint inhibitors (ICIs), which promote or restore antitumour immunity by inhibiting immunosuppressive signalling pathways, are currently the most widely used immunotherapies in these patients. However, immune-related adverse events (irAEs) or disease progression often necessitate discontinuation of these agents, leaving many patients with limited subsequent treatment options. In this scenario, ICI rechallenge has emerged as a potential strategy. Despite this potential, evidence for ICI rechallenge after either disease progression or irAEs in patients with non-small-cell lung cancer is limited and evidence for those with small cell lung cancer seems to be non-existent. In this Review, we provide a comprehensive overview of the available data on ICI rechallenge in the context of both disease progression and irAEs, including a summary of current guidance on clinical management and detailed discussions of safety and efficacy. We also highlight important unanswered questions in an attempt to guide future research in this area. Patients with advanced-stage lung cancer might discontinue immune-checkpoint inhibitor (ICI) treatment for various reasons, including toxicities, disease progression or disease remission. Nonetheless, treatment options in this setting are often limited and some patients might derive benefit from re-administration of a previously received ICI. In this Review, the authors summarize the available data on ICI rechallenge, including the reasons for discontinuation and the feasibility of rechallenge in various clinical scenarios, and highlight important unaddressed research questions.
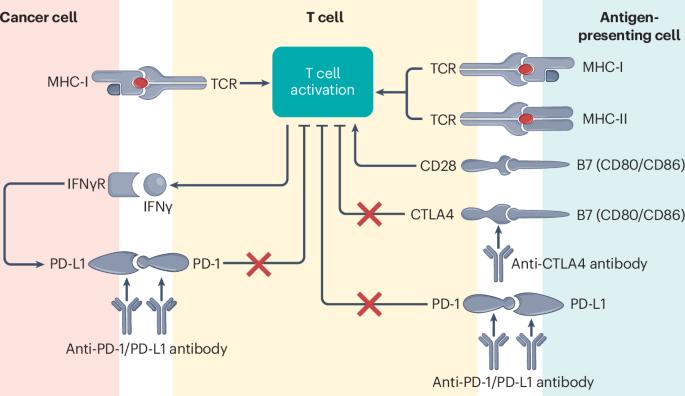

免疫检查点抑制剂在晚期肺癌患者中的再挑战
肺癌仍然是全球癌症相关死亡的主要原因,许多患者被诊断为晚期疾病。这种情况下的治疗依赖于全身治疗,包括化疗、靶向治疗和免疫治疗。免疫检查点抑制剂(ICIs)通过抑制免疫抑制信号通路来促进或恢复抗肿瘤免疫,是目前在这些患者中使用最广泛的免疫疗法。然而,免疫相关不良事件(irAEs)或疾病进展往往需要停用这些药物,使许多患者的后续治疗选择有限。在这种情况下,ICI重新挑战已成为一种潜在的战略。尽管有这种潜力,但非小细胞肺癌患者在疾病进展或irae后ICI再挑战的证据有限,而小细胞肺癌患者的证据似乎不存在。在这篇综述中,我们提供了在疾病进展和irae背景下ICI再挑战的现有数据的全面概述,包括当前临床管理指南的总结以及对安全性和有效性的详细讨论。我们还强调了一些重要的未解问题,试图指导该领域未来的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


