Chlorine-facilitated amorphous Co-based catalyst with self-termination of surface reconstruction for seawater splitting
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
A highly selective and durable electrocatalyst for the oxygen evolution reaction (OER) is essential for effective seawater splitting, as the side reactions induced by Cl– ions pose a significant challenge. Employing OER catalysts with excellent crystallinity allows for a thorough investigation of the dynamic restructuring in active sites. Nevertheless, the specific mechanism of reconstruction triggered by the amorphous phase remains unclear. Herein, we integrate simultaneously amorphous structures and lattice chlorine components into Co-based electrocatalysts (Cl-Co/CoOx-p@GF), enabling us to investigate how lattice chlorine affects the reconstruction mechanism of a highly amorphous structure. Combining in-situ and ex-situ characterizations, we find that the amorphous cobalt oxides undergo re-crystallization, resulting in irreversible surface reconstruction with a terminal layer due to structural flexibility during seawater splitting. Notably, the introduced lattice chlorine acts as a structural buffer, whose sustained leaching during OER can create vacancies for seawater Cl− invasion, thereby preventing catalyst deactivation. Additionally, depending on the photosensitivity of porphyrin-based structural carriers, the Cl-Co/CoOx-p@GF demonstrates an overpotential of 385 mV vs. RHE at 100 mA cm–2 while maintaining a current density of 95.4 % over 48 h with the assistance of visible light in alkaline simulated seawater.
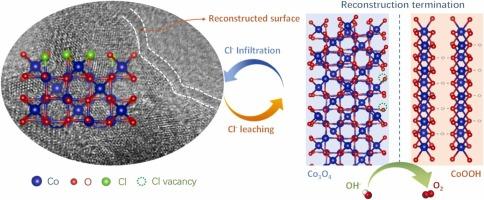
具有自终止表面重构的氯促进非晶态co基催化剂用于海水分裂
高选择性和耐用的析氧反应电催化剂是有效分解海水的必要条件,因为氯离子引起的副反应是一个重大挑战。采用具有优异结晶度的OER催化剂,可以对活性位点的动态重组进行彻底的研究。然而,由非晶相引发的重构的具体机制尚不清楚。在此,我们将非晶结构和晶格氯组分同时集成到co基电催化剂(Cl-Co/CoOx-p@GF)中,使我们能够研究晶格氯如何影响高度非晶结构的重建机制。结合原位和非原位表征,我们发现无定形钴氧化物在海水分裂过程中由于结构柔韧性而发生再结晶,导致具有终端层的不可逆表面重构。值得注意的是,引入的晶格氯作为结构缓冲剂,其在OER过程中的持续浸出可以为海水Cl−入侵创造空位,从而防止催化剂失活。此外,根据基于卟啉的结构载体的光敏性,Cl-Co/CoOx-p@GF在碱性模拟海水中,在可见光的帮助下,在100 mA cm-2下,相对于RHE的过电位为385 mV,同时在48小时内保持95.4%的电流密度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


