2D nonlayered titanium oxynitrides intercalated with nitrogen-doped graphene for pseudocapacitive energy storage
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
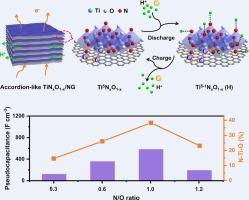
Nanostructured titanium oxynitride (TiNxO1-x) is a promising pseudocapacitive material due to its large pseudocapacitance and high conductivity. However, the synthesis of nonlayered two-dimensional (2D) TiNxO1-x is challenging and the pseudocapacitive energy storage mechanism of TiNxO1-x is still unclear. Herein, accordion-like 2D TiNxO1-x nanosheets intercalated with nitrogen-doped graphene (TiNxO1-x/NG) are controllably fabricated by a spatially confined thermal nitridation of 1,8-diaminooctane intercalated H0.8Ni0.4Ti1.6O4·H2O nanosheets. In situ electrochemical characterizations and theoretical calculations reveal the pseudocapacitance originates from the Faradic reaction between H+ and N![]() Ti
Ti![]() O species accompanied by Ti valence state change and the pseudocapacitance of TiNxO1-x/NG is determined by the N
O species accompanied by Ti valence state change and the pseudocapacitance of TiNxO1-x/NG is determined by the N![]() Ti
Ti![]() O content instead of the N/O ratio. Compared to TiNxO1-x, the electrons transfer from sandwiched NG to TiNxO1-x enhances H+ adsorption at N active sites of N
O content instead of the N/O ratio. Compared to TiNxO1-x, the electrons transfer from sandwiched NG to TiNxO1-x enhances H+ adsorption at N active sites of N![]() Ti
Ti![]() O within TiNxO1-x/NG, giving rise to enhanced pseudocapacitance and cycling stability. Consequently, the TiNxO1-x/NG shows a large volumetric capacitance of 739.2 F cm−3 (616.0 F g−1) at 1 A cm−3 and superior cyclability with 98.5 % capacitance retention after 10,000 cycles, surpassing previously reported metal oxynitrides and nitrides. The results provide insights into the controllable synthesis of 2D nonlayered TiNxO1-x and elucidate the pseudocapacitive charge storage mechanism of metal nitrides or oxynitrides.
O within TiNxO1-x/NG, giving rise to enhanced pseudocapacitance and cycling stability. Consequently, the TiNxO1-x/NG shows a large volumetric capacitance of 739.2 F cm−3 (616.0 F g−1) at 1 A cm−3 and superior cyclability with 98.5 % capacitance retention after 10,000 cycles, surpassing previously reported metal oxynitrides and nitrides. The results provide insights into the controllable synthesis of 2D nonlayered TiNxO1-x and elucidate the pseudocapacitive charge storage mechanism of metal nitrides or oxynitrides.

氮掺杂石墨烯的二维非层状氮化钛赝电容储能
纳米氧化氮化钛(TiNxO1-x)具有大赝电容和高导电性,是一种很有前途的赝电容材料。然而,非层状二维(2D) TiNxO1-x的合成具有挑战性,TiNxO1-x的赝电容储能机制尚不清楚。本文通过对1,8-二氨基辛烷嵌层的H0.8Ni0.4Ti1.6O4·H2O纳米片进行空间限制热氮化,制备了嵌层氮掺杂石墨烯(TiNxO1-x/NG)的手风琴状二维TiNxO1-x纳米片。原位电化学表征和理论计算表明,TiNxO1-x/NG的赝电容来源于H+和NTiO之间的法拉奇反应,并伴随着Ti价态的变化,其赝电容由NTiO含量决定,而不是由N/O比决定。与TiNxO1-x相比,从夹心NG到TiNxO1-x的电子转移增强了TiNxO1-x/NG中NTiO N活性位点上H+的吸附,从而增强了赝电容和循环稳定性。因此,TiNxO1-x/NG在1 a cm−3时显示出739.2 F cm−3(616.0 F g−1)的大容量电容,并且在10,000次 次循环后具有98.5% %的优异可循环性,超过了先前报道的金属氧氮化物和氮化物。研究结果为二维非层状TiNxO1-x的可控合成提供了新的思路,并阐明了金属氮化物或氧氮化物的赝电容电荷存储机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


