In situ structure of the mouse sperm central apparatus reveals mechanistic insights into asthenozoospermia
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
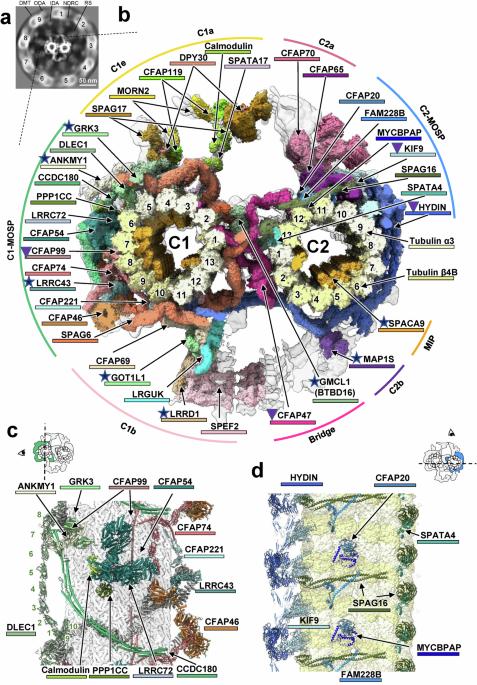
The central apparatus (CA) within the sperm axoneme is vital for sperm motility, yet its molecular architecture and functional mechanisms remain incompletely understood. Combining cryo-electron tomography and AlphaFold2, we resolved the in-cell structure of mouse sperm CA at a subnanometer resolution and built a near-complete atomic model. Our analysis identified 39 CA-associated proteins, including eight previously unreported components. By presenting the full-length structures of CFAP47 and HYDIN, we elucidate their molecular roles in tethering the C1 and C2 microtubules within the CA. Specifically, HYDIN forms a semicircular chain that encircles C1 and C2, with its N-terminal half driving the C1–C2 connection and its C-terminal half providing axial support in C2. CFAP47, the core structural component of the bridge, binds C1 through its N-terminal domains, interacts with HYDIN via its central CFAP47-ring, and anchors to C2 through its C-terminal region. The significantly reduced sperm motility and impaired CA structure observed in Cfap47-knockout mice confirmed the important role of CFAP47. Furthermore, genetic analysis of infertile Chinese men with asthenozoospermia identified previously unreported mutations in the CFAP47. The CA structural model elucidates the pathogenic mechanisms of these mutations, establishing a direct link between CFAP47 dysfunction and impaired sperm motility. Therefore, our study provides mechanistic insights into CA-related fertility disorders.
小鼠精子中心装置的原位结构揭示了弱精子症的机制见解。
精子轴素内的中央装置(CA)对精子的运动至关重要,但其分子结构和功能机制尚不完全清楚。结合冷冻电子断层扫描和AlphaFold2,我们在亚纳米分辨率下解析了小鼠精子CA的细胞内结构,并建立了一个接近完整的原子模型。我们的分析鉴定了39种ca相关蛋白,包括8种以前未报道的成分。通过展示CFAP47和HYDIN的全长结构,我们阐明了它们在CA内拴住C1和C2微管的分子作用。具体来说,HYDIN形成一个围绕C1和C2的半圆形链,其n端一半驱动C1-C2连接,其c端一半在C2中提供轴向支撑。CFAP47是该桥的核心结构成分,通过其n端结构域结合C1,通过其中心CFAP47环与HYDIN相互作用,并通过其c端区域锚定到C2。在CFAP47敲除小鼠中观察到的精子活力显著降低和CA结构受损证实了CFAP47的重要作用。此外,对患有弱精子症的不育中国男性的遗传分析发现了此前未报道的CFAP47突变。CA结构模型阐明了这些突变的致病机制,建立了CFAP47功能障碍与精子活力受损之间的直接联系。因此,我们的研究为ca相关的生育障碍提供了机制见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


