Synthesis of SeCF3-Substituted Oxazines and Oxazolines through Electrophilic Trifluoromethylselenolation Cyclization of Propargylic Amides.
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-06-20
Epub Date: 2025-06-05
DOI:10.1021/acs.joc.5c00930
引用次数: 0
Abstract
The electrophilic trifluoromethylselenolation cyclizations of propargylic amides are disclosed. The transformations undergo the 6-endo-dig and 5-exo-dig cyclizations to synthesize SeCF3-substituted oxazines and oxazolines, respectively, which are induced by N-trifluoromethylselenophthalimide. This protocol is suitable for late-stage applications of complex natural products and drug molecules.
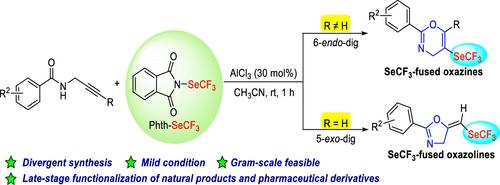
丙炔酰胺亲电三氟甲基硒化环化合成secf3取代的恶嗪和恶唑啉。
公开了丙炔酰胺的亲电三氟甲基硒化环化。n -三氟甲基硒眼酰亚胺分别通过6-内切环和5-外切环合成secf3取代的恶嗪和恶唑啉。该方案适用于复杂天然产物和药物分子的后期应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


