Vimentin network dysregulation mediates neurite deficits in SNCA duplication Parkinson’s patient–derived midbrain neurons
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
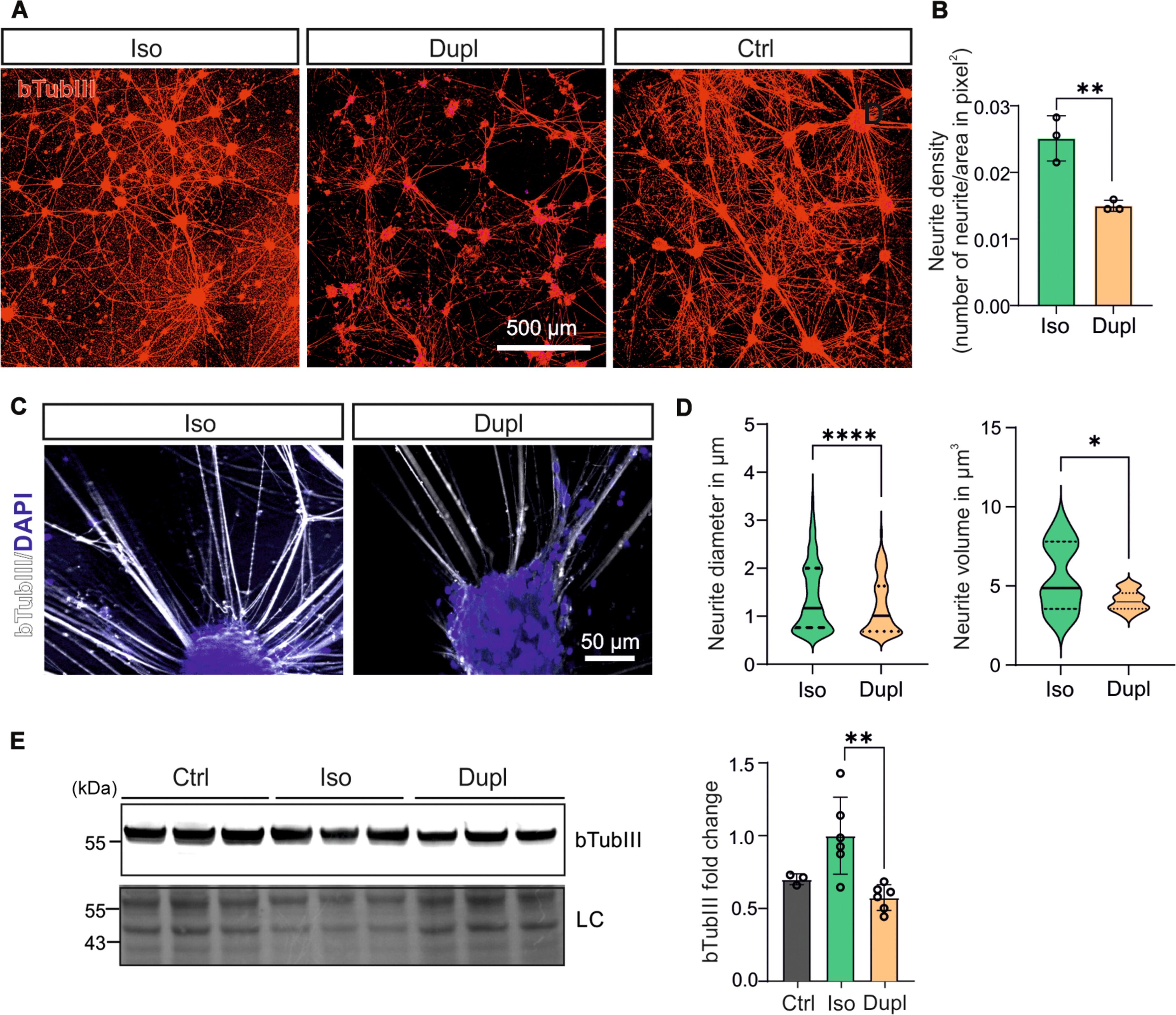
Duplication of the SNCA gene (SNCADupl), linked to elevated levels of α-synuclein (aSyn), is a genetic cause of Parkinson’s disease (PD). Our prior work with human-induced pluripotent stem cell (hiPSC)–derived midbrain neurons generated from patients with PD SNCADupl identified neuritic deficits, accompanied by decreased levels of cytoskeletal element β-tubulin-III (bTubIII). To explore mechanisms underlying these effects in SNCADupl neurons, we used CRISPR-Cas9 to generate isogenic control hiPSCs. Isogenic correction of SNCA dosage restored SNCADupl-induced neurite defects and bTubIII levels. Multi-omics analyses revealed SNCADupl-induced alterations in neuronal differentiation, with a notable down-regulation of PAX6. Moreover, SNCADupl induced an up-regulation of vimentin. Further characterization revealed heightened vimentin truncation associated with altered distribution and organization. Similar changes in vimentin levels and truncation were observed in postmortem putamen tissue from patients with sporadic PD. Notably, targeting vimentin with okadaic acid and withaferin A restored bTubIII- and neurite-associated defects, suggesting its potential to prevent aSyn-mediated neuritic degeneration.
Vimentin网络失调介导SNCA复制帕金森患者衍生的中脑神经元的神经突缺陷
SNCA基因(SNCADupl)的重复与α-突触核蛋白(aSyn)水平升高有关,是帕金森病(PD)的遗传原因。我们之前对PD SNCADupl患者产生的人诱导多能干细胞(hiPSC)来源的中脑神经元的研究发现,神经缺损伴随着细胞骨架元件β-微管蛋白- iii (bTubIII)水平的降低。为了探索SNCADupl神经元中这些作用的机制,我们使用CRISPR-Cas9生成等基因对照hiPSCs。SNCA剂量的等基因校正恢复了sncadup诱导的神经突缺陷和bTubIII水平。多组学分析显示sncadup诱导神经元分化改变,PAX6显著下调。此外,SNCADupl诱导了vimentin的上调。进一步的表征表明,与分布和组织改变有关的波形蛋白截断增加。在散发性PD患者的死后壳核组织中观察到类似的波形蛋白水平变化和截短。值得注意的是,用okadaic酸和withaferin A靶向vimentin可以恢复bTubIII和神经ite相关缺陷,这表明它有可能预防async介导的神经性变性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


