Exploring the anti-inflammatory effects of total flavonoids from L. gracile on LPS-induced inflammation: An integrated approach combining network pharmacology, molecular docking, and experimental validation
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
Abstract
Background
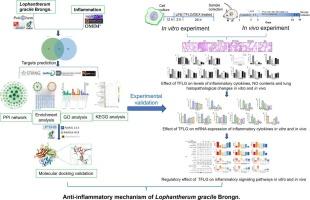
Lophatherum gracile Brongn., also known as Dan-Zhu-Ye, is traditionally used to clear heat and purge fire, relieve irritability and thirst, diuresis and catharsis. Our preliminary research revealed that total flavonoid extracts from Lophantherum gracile Brongn. (TFLG) can reverse the inflammatory response induced by LPS in mice, but the exact mechanism is still unclear.
Methods
The main chemical constituents of TFLG were first analyzed via liquid chromatography tandem high-resolution mass spectrometry (LC-HRMS). We then used network pharmacology to screen potential targets of TFLG to treat inflammation. Subsequently, inflammatory models were established in 3D4/2 cells and SPF Kunming mice via lipopolysaccharide. The predicted potential targets and associated signaling pathways were further validated through ELISA, western blot analysis, and qRT-PCR.
Results
The LC-HRMS results revealed that approximately 36 compounds with contents greater than 0.1 % were identified in TFLG. Network pharmacology analysis revealed that 103 common targets of TFLG are associated with inflammation. The results of molecular docking indicated that the main ingredients of L. gracile (quercetin, kaempferol, and luteolin) can exert anti-inflammatory effects through binding with the inflammatory targets TNF, IL-6, and IL-1β. Our experimental results demonstrated that TFLG reversed the upregulation of IL-6, IL-1β, IL-12, and TNF-α and the downregulation of IL-10 in both in vitro and in vivo inflammatory models induced by LPS. The qRT-PCR results were consistent with the ELISA results above. Western blot analysis revealed that TFLG reduced the expression levels of p65, p-p65, p-IκB-α, p38, and p-p38 and increased the expression of IκB-α.
Conclusions
TFLG can exert an anti-inflammatory effect by inhibiting the NF-κB and MAPK signaling pathways, which aligns with the anticipated outcomes of network pharmacology. This study offers new evidence supporting the potential application of TFLG as a medication for reducing inflammation.
网络药理学、分子对接、实验验证相结合的方法探讨细叶总黄酮对lps诱导炎症的抗炎作用
背景:长柄草。也被称为丹竹叶,传统上用于清热泻火,缓解烦躁和口渴,利尿和通便。初步研究发现,细棘棘总黄酮提取物具有一定的生物活性。(TFLG)可以逆转LPS诱导的小鼠炎症反应,但其确切机制尚不清楚。方法采用液相色谱串联高分辨率质谱法(LC-HRMS)分析其主要化学成分。然后,我们使用网络药理学筛选TFLG治疗炎症的潜在靶点。随后,通过脂多糖在3D4/2细胞和SPF昆明小鼠中建立炎症模型。预测的潜在靶点和相关信号通路通过ELISA、western blot分析和qRT-PCR进一步验证。结果hplc - hrms鉴定出含量大于0.1%的化合物约36个。网络药理学分析显示,TFLG的103个共同靶点与炎症有关。分子对接结果表明,槲皮素、山奈酚、木犀草素等主要成分可通过与炎症靶点TNF、IL-6、IL-1β结合发挥抗炎作用。我们的实验结果表明,在LPS诱导的体内和体外炎症模型中,TFLG逆转了IL-6、IL-1β、IL-12和TNF-α的上调和IL-10的下调。qRT-PCR结果与上述ELISA结果一致。Western blot分析显示,TFLG降低了p65、p-p65、p- κ b -α、p38和p-p38的表达水平,增加了i - κ b -α的表达。结论stflg通过抑制NF-κB和MAPK信号通路发挥抗炎作用,符合网络药理学的预期结果。本研究为TFLG作为消炎药物的潜在应用提供了新的证据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


