Efficacy of regular prophylaxis with a plasma-derived von Willebrand factor/factor VIII concentrate with a 1:1 activity ratio in reducing heavy menstrual bleeding in girls/women with von Willebrand disease
引用次数: 0
Abstract
Background
Heavy menstrual bleeding is the dominant symptom in girls/women with von Willebrand disease (VWD), affecting ∼80 to 90%. Female patients with VWD are typically diagnosed later than males, despite the disproportionate impact of heavy menstrual bleeding. Although heavy menstrual bleeding has a substantial impact on patients, it is commonly undertreated in part because of the potential multifactorial causes in young girls/women, imprecise definition, and exclusion of this bleeding type as an indication for prophylaxis in previous studies. The WIL-31 study showed that prophylaxis with wilate (a plasma-derived von Willebrand factor/factor VIII concentrate with a 1:1 activity ratio) is highly efficacious in reducing bleeding rates in adults and children with VWD of all types. The impact of wilate prophylaxis on heavy menstrual bleeding was evaluated as an exploratory endpoint since it was not included as part of the primary endpoint.
Objective(s)
To investigate the efficacy of regular prophylaxis with wilate in reducing the incidence of heavy menstrual bleeding in girls/women with VWD who had previously been treated on-demand.
Study design
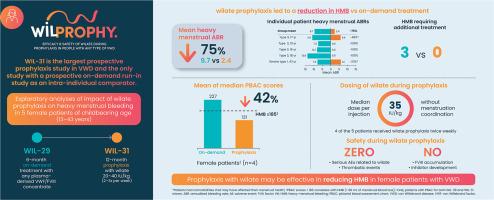
WIL-31 (NCT04052698) was a prospective, non-controlled, international, multicenter Phase 3 trial that enrolled male/female patients, aged ≥ 6 years old with VWD. Prior to entering the WIL-31 study, all patients received on-demand treatment with a von Willebrand factor-containing product during a 6-month, prospective, observational, run-in study (WIL-29). Patients in WIL-31 received wilate prophylaxis 2–3 times per week at a dose of 20–40 IU/kg for 12 months. Prophylaxis was not tailored to time with menstruation. Heavy menstrual bleeding was defined as any menstrual bleed that impeded the ability to perform daily activities during menstrual periods. Criteria for heavy menstrual bleeding included changing pads more frequently than hourly, menstrual bleeding lasting 7 or more days, or the presence of clots > 1 cm, combined with a history of flooding or a Pictorial Blood Assessment Chart score ≥ 185.
Results
Of the 33 patients evaluated in the study, 14 (42%) were female, of whom 5 (15%) were of childbearing age (13–43 years old). One of these had VWD type 1, the other 4 had VWD type 3. Prophylaxis with wilate reduced the mean annualized heavy menstrual bleeding rate by 75% compared with on-demand treatment (2.4 vs 9.7 in WIL-31 and WIL-29, respectively). During 6 months of on-demand treatment, all 5 female patients experienced at least 1 heavy menstrual bleed, whereas during 12 months of prophylaxis, 3 (60%) experienced no heavy menstrual bleeding episodes. During on-demand treatment, 5 patients experienced 26 heavy menstrual bleeding episodes, 3 (12%) of which required additional treatment. Under prophylaxis, 2 patients experienced 12 heavy menstrual bleeding episodes, of which only 1 (8%) impeded daily activity and none required additional treatment.
Conclusions
In these exploratory analyses, wilate prophylaxis given 2–3 times a week without menstruation coordination was efficacious in reducing heavy menstrual bleeding in girls/women with VWD compared with on-demand treatment. Long-term prophylaxis has the potential to play a major role in improving the care and reducing the disease burden for girls/women with VWD.
血浆源性血管性血友病因子/因子VIII浓缩物1:1活性比定期预防血管性血友病女孩/妇女月经大量出血的疗效
大量月经出血是患有血管性血友病(VWD)的女孩/妇女的主要症状,约占80%至90%。尽管月经大量出血的影响不成比例,但女性VWD患者的诊断通常比男性晚。尽管大量月经出血对患者有重大影响,但通常治疗不足,部分原因是年轻女孩/妇女中潜在的多因素原因,不精确的定义,以及在以前的研究中排除了这种出血类型作为预防的指征。will -31研究表明,预防使用wilate(血浆来源的血管性血友病因子/ VIII因子浓缩物,活性比为1:1)在降低所有类型VWD的成人和儿童出血率方面非常有效。wilate预防对大量月经出血的影响作为一个探索性终点进行评估,因为它没有被纳入主要终点的一部分。目的(s)调查wilate常规预防在减少以前按需治疗的VWD女孩/妇女的大量月经出血发生率方面的疗效。研究设计:will -31 (NCT04052698)是一项前瞻性、非对照、国际、多中心的3期临床试验,纳入年龄≥6岁的VWD患者。在进入wil31研究之前,所有患者在为期6个月的前瞻性、观察性、磨合研究(wil29)中接受了含血管性血友病因子产品的按需治疗。wili -31患者每周接受2-3次wilate预防,剂量为20-40 IU/kg,持续12个月。预防措施并不是根据月经时间而定的。大量月经出血被定义为月经期间妨碍日常活动的任何月经出血。大量月经出血的标准包括更换卫生巾的频率超过每小时,月经出血持续7天或更长时间,或存在凝块。1 cm,合并有泛洪病史或血画报评分≥185。结果33例患者中,女性14例(42%),育龄5例(15%)(13-43岁)。其中一人患有1型VWD,另外4人患有3型VWD。与按需治疗相比,wilate预防使平均年化月经大出血率降低了75%(分别为2.4 vs 9.7,分别为will -31和will -29)。在按需治疗的6个月期间,所有5名女性患者都经历了至少1次大量月经出血,而在12个月的预防期间,3名(60%)患者没有经历大量月经出血。在按需治疗期间,5例患者经历了26次重度月经出血,其中3例(12%)需要额外治疗。在预防治疗下,2例患者经历了12次严重月经出血,其中只有1例(8%)妨碍了日常活动,没有人需要额外的治疗。结论在这些探索性分析中,与按需治疗相比,每周给予2-3次无月经协调的预防治疗可有效减少VWD女孩/妇女的大量月经出血。长期预防有可能在改善对患有寨卡病毒病的女孩/妇女的护理和减轻疾病负担方面发挥重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

AJOG global reports
Endocrinology, Diabetes and Metabolism, Obstetrics, Gynecology and Women's Health, Perinatology, Pediatrics and Child Health, Urology
CiteScore
1.20
自引率
0.00%
发文量
0
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


