Safety and efficacy of alcohol-mediated bilateral adrenal artery embolization in patients with idiopathic hyperaldosteronism: a 6-month follow-up of a randomized controlled trial
IF 3.4
4区 医学
Q2 PERIPHERAL VASCULAR DISEASE
引用次数: 0
Abstract
Although unilateral adrenal artery embolization (AAE) has emerged as an alternative treatment for patients with primary aldosteronism (PA), it may be insufficient for treating patients with idiopathic hyperaldosteronism (IHA) due to the bilateral nature of their condition. This study aimed to investigate the safety and efficacy of alcohol-mediated bilateral adrenal artery embolization (Bi-AAE) in patients with idiopathic hyperaldosteronism (IHA). A total of 72 patients were randomly assigned in a (1:1) ratio to receive either Bi-AAE or spironolactone (20–60 mg/day). The primary endpoint was the change in office systolic blood pressure (SBP) from baseline to 6 months. Key secondary endpoints included changes in 24 h blood pressure, aldosterone levels, aldosterone-to-renin ratio (ARR), and serum potassium. At 6 months, Bi-AAE significantly reduced office SBP compared to spironolactone (−18.9 ± 16.4 mmHg vs. −11.6 ± 9.3 mmHg; treatment difference: −7.4 mmHg; P = 0.03), with a greater proportion of Bi-AAE patients achieving target SBP (<140 mmHg; 77.1% vs. 51.5%; P = 0.027). Bi-AAE also resulted in significantly greater reductions in 24 h and home SBP at 1, 3, and 6 months (all P < 0.05). Furthermore, Bi-AAE was more effective in correcting biochemical abnormalities, including hyperaldosteronism and renin suppression (all P < 0.05). Importantly, Bi-AAE preserved zona fasciculata function, as evidenced by normal morning serum cortisol levels and intact responses to ACTH stimulation post-procedure. No serious adverse events occurred during the perioperative or 6-month follow-up period. These findings support Bi-AAE as a safe, minimally invasive, and highly effective alternative to medical therapy for managing IHA. Although the findings support Bi-AAE as a safe, minimally invasive, and highly effective alternative to medical therapy for managing IHA, the need for long-term data before drawing definitive conclusions is emphasized. Future studies with extended follow-up are necessary to confirm its long-term benefits and risks. Trial registration: The trial has been registered at ClinicalTrials.gov (NCT05262660).
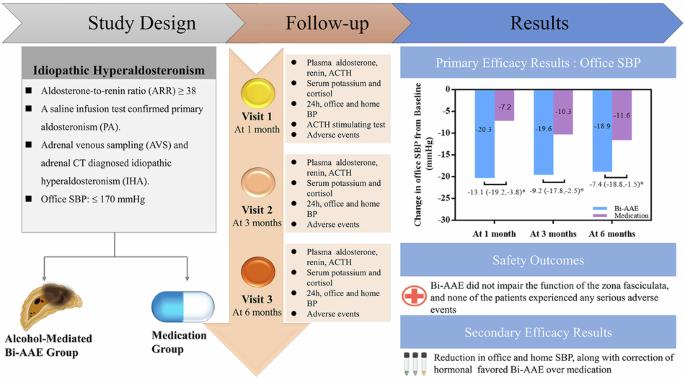
酒精介导双侧肾上腺动脉栓塞治疗特发性高醛固酮增多症患者的安全性和有效性:一项为期6个月的随机对照试验随访
虽然单侧肾上腺动脉栓塞(AAE)已成为原发性醛固酮增多症(PA)患者的替代治疗方法,但由于其双侧性,它可能不足以治疗特发性高醛固酮增多症(IHA)患者。本研究旨在探讨酒精介导的双侧肾上腺动脉栓塞(Bi-AAE)治疗特发性高醛固酮增多症(IHA)患者的安全性和有效性。共有72例患者按1:1的比例随机分配接受双aae或螺内酯(20-60 mg/天)治疗。主要终点是从基线到6个月的办公室收缩压(SBP)变化。关键次要终点包括24小时血压、醛固酮水平、醛固酮与肾素比值(ARR)和血清钾的变化。6个月时,与螺内酯相比,Bi-AAE显著降低办公室收缩压(-18.9±16.4 mmHg vs -11.6±9.3 mmHg;治疗差异:-7.4 mmHg;P = 0.03), Bi-AAE患者达到目标收缩压的比例更高(
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Human Hypertension
医学-外周血管病
CiteScore
5.20
自引率
3.70%
发文量
126
审稿时长
6-12 weeks
期刊介绍:
Journal of Human Hypertension is published monthly and is of interest to health care professionals who deal with hypertension (specialists, internists, primary care physicians) and public health workers. We believe that our patients benefit from robust scientific data that are based on well conducted clinical trials. We also believe that basic sciences are the foundations on which we build our knowledge of clinical conditions and their management. Towards this end, although we are primarily a clinical based journal, we also welcome suitable basic sciences studies that promote our understanding of human hypertension.
The journal aims to perform the dual role of increasing knowledge in the field of high blood pressure as well as improving the standard of care of patients. The editors will consider for publication all suitable papers dealing directly or indirectly with clinical aspects of hypertension, including but not limited to epidemiology, pathophysiology, therapeutics and basic sciences involving human subjects or tissues. We also consider papers from all specialties such as ophthalmology, cardiology, nephrology, obstetrics and stroke medicine that deal with the various aspects of hypertension and its complications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


