Efficacy and safety of PCSK9 monoclonal antibodies in older patients: A real-world registry
IF 5.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background and aims
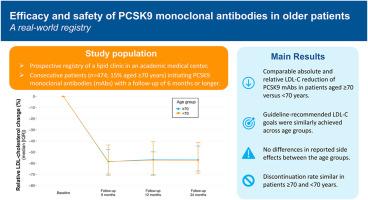
Proprotein convertase subtilisin/kexin type 9 monoclonal antibodies (PCSK9 mAbs) have a favourable efficacy and safety profile. Advancing age may pose challenges such as increasing drug toxicity, polypharmacy, and comorbidities. This study aims to assess whether the efficacy and safety of PCSK9 mAbs are comparable between patients ≥70 years versus patients <70 years.
Methods
In a prospective registry of all consecutive patients who started PCSK9 mAbs as part of routine care in a university medical center-based lipid clinic, data was collected on LDL cholesterol levels, side effects, and discontinuation. Data on efficacy and safety (reported side effects and discontinuation) were stratified for patients ≥70 and < 70 years.
Results
Of the 474 patients (median age 59 [51–66] years, 51 % men) who started a PCSK9 mAb, 70 patients were ≥70 years (15 %). After 6 months, relative and absolute LDL cholesterol reduction was similar across age groups (relative decrease: 58 % [48–70] vs 59 % [44–71], p = 0.99; mean (SD) absolute decrease 2.4 (0.8) vs 2.4 (1.2) mmol/L, p = 0.90). A comparable proportion of patients ≥70 years compared to those <70 years achieved European and Dutch guideline-recommended goals (36 % vs 46 %, p = 0.18, and 54 % vs 62 %, p = 0.26, respectively). Efficacy outcomes were similar after 12 and 24 months follow-up. Reported side effects and discontinuation of PCSK9 mAbs were comparable across age groups.
Conclusions
Efficacy and safety of PCSK9 mAbs are comparable for patients ≥70 years and patients <70 years in a real-world study.
PCSK9单克隆抗体在老年患者中的有效性和安全性:一个真实世界的注册表。
背景与目的:Proprotein convertase subtilisin/kexin 9型单克隆抗体(PCSK9 mab)具有良好的疗效和安全性。年龄的增长可能带来挑战,如增加药物毒性,多种药物和合并症。本研究旨在评估PCSK9单克隆抗体在≥70岁患者与患者之间的疗效和安全性是否具有可比性。方法:在一所大学医学中心的脂质诊所,对所有连续开始使用PCSK9单克隆抗体作为常规护理的患者进行前瞻性登记,收集LDL胆固醇水平、副作用和停药的数据。对≥70岁的患者进行疗效和安全性(报告的副作用和停药)数据分层。结果:在474例开始使用PCSK9单抗的患者(中位年龄59[51-66]岁,51%为男性)中,70例患者年龄≥70岁(15%)。6个月后,各年龄组LDL胆固醇的相对和绝对降低率相似(相对降低:58% [48-70]vs 59% [44-71], p = 0.99;平均(SD)绝对下降2.4 (0.8)vs 2.4 (1.2) mmol/L, p = 0.90)。结论:PCSK9单抗在≥70岁患者和患者中的疗效和安全性是相当的
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Atherosclerosis
医学-外周血管病
CiteScore
9.80
自引率
3.80%
发文量
1269
审稿时长
36 days
期刊介绍:
Atherosclerosis has an open access mirror journal Atherosclerosis: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Atherosclerosis brings together, from all sources, papers concerned with investigation on atherosclerosis, its risk factors and clinical manifestations. Atherosclerosis covers basic and translational, clinical and population research approaches to arterial and vascular biology and disease, as well as their risk factors including: disturbances of lipid and lipoprotein metabolism, diabetes and hypertension, thrombosis, and inflammation. The Editors are interested in original or review papers dealing with the pathogenesis, environmental, genetic and epigenetic basis, diagnosis or treatment of atherosclerosis and related diseases as well as their risk factors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


