Effect of NH4NO3 pre-treatment on hydrogen adsorption properties of Na+, K+, Ag+, Li+, H+, Ni2+, Ca2+, Cu2+ and Mg2+ exchanged natural clinoptilolite
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The objective of this study was to investigate the effect of NH4NO3 modification and calcination pretreatment on the hydrogen adsorption properties of Gördes clinoptilolite (CLN). The H+ form (H-CLN) was obtained by modification with 1.0 M NH4NO3 for 12 h followed by calcining at 450 °C for 6 h. The cation-exchanged samples of the H-CLN were obtained with 0.1 and 0.5 M of NaNO3, KNO3, LiNO3, AgNO3, Ni(NO3)2, Ca(NO3)2, Cu(NO3)2 and Mg(NO3)2 solutions. XRD analysis demonstrated that the pretreatment used did not lead to the loss of the crystalline structure of the samples. XRF results indicated a slight increase in the SiO2/Al2O3 ratio, from 5.35 for CLN to 5.45–5.84 for H-CLN and its cation-exchanged forms. The BET surface areas (35.68–259.18 m2 g−1) and hydrogen adsorption capacities of the CLNs (0.244–0.726 wt% at 77 K) demonstrated that the applied NH4NO3 exchange and calcination pretreatment markedly enhanced the textural and gas adsorption properties. The 0.5-Li-CLN exhibited the highest hydrogen adsorption capacity of 0.726 wt%.
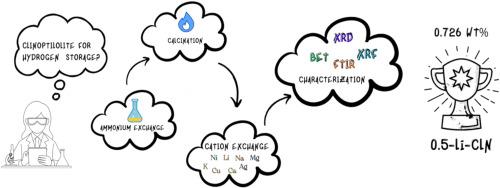
NH4NO3预处理对Na+、K+、Ag+、Li+、H+、Ni2+、Ca2+、Cu2+、Mg2+交换性天然斜沸石吸氢性能的影响
研究了NH4NO3改性和煅烧预处理对Gördes斜沸石(CLN)吸氢性能的影响。用1.0 M NH4NO3改性12 H, 450℃煅烧6 H,得到H+形态的H- cln,用0.1 M和0.5 M的NaNO3、KNO3、LiNO3、AgNO3、Ni(NO3)2、Ca(NO3)2、Cu(NO3)2和Mg(NO3)2溶液进行阳离子交换得到H- cln。XRD分析表明,所采用的预处理并没有导致样品晶体结构的损失。XRF结果表明,CLN的SiO2/Al2O3比值略有增加,从CLN的5.35增加到H-CLN及其阳离子交换形式的5.45-5.84。在77 K时,CLNs的BET表面积(35.68 ~ 259.18 m2 g−1)和氢气吸附量(0.244 ~ 0.726 wt%)表明,NH4NO3交换和煅烧预处理显著提高了CLNs的结构和气体吸附性能。0.5-Li-CLN的吸氢量最高,为0.726 wt%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


