A dual-targeting strategy to inhibit colorectal cancer liver metastasis via tumor cell ferroptosis and cancer-associated fibroblast reprogramming
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Colorectal cancer (CRC) remains a leading cause of cancer‐related mortality, with liver metastasis posing a significant therapeutic challenge. Within the “seed and soil” paradigm, disrupting both tumor cells and their supportive microenvironment is essential to suppress disease progression. Here, we utilized single‐cell transcriptomics of clinical CRC samples identified NOX4+ (NADPH oxidase 4 positive) cancer‐associated fibroblasts (CAFs) and CXCR4+ (C-X-C motif chemokine receptor 4 positive)/GPX4+ (glutathione peroxidase 4 positive) tumor cells as critical drivers of metastasis. Consequently, a dual‐targeted nanosystem was thus devised to induce ferroptosis in tumor cells and reprogram CAFs. This strategy integrates a ferroptosis inducer encapsulated within the cancer cell membrane and a CXCR4–NOX4 inhibitor loaded onto a hybrid membrane composed of cancer cells and CAFs, thereby achieving dual synergistic effects: ferroptotic eradication of malignant cells and induction of CAFs quiescence. In orthotopic, liver metastasis, and patient-derived tumor xenograft humanized immune mouse models, these nanoparticles significantly suppressed tumor growth, mitigated immunosuppressive signaling, and augmented antitumor immune responses, while maintaining favorable biocompatibility. These findings highlight the potential of simultaneously targeting ferroptosis in tumor cells and CAFs reprogramming in the tumor microenvironment to overcome liver metastasis of CRC.
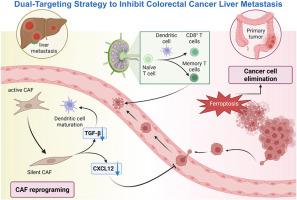
通过肿瘤细胞铁下垂和癌症相关成纤维细胞重编程抑制结直肠癌肝转移的双靶向策略
结直肠癌(CRC)仍然是癌症相关死亡的主要原因,肝转移给治疗带来了重大挑战。在“种子和土壤”模式下,破坏肿瘤细胞及其支持性微环境对于抑制疾病进展至关重要。在这里,我们利用临床CRC样本的单细胞转录组学发现NOX4+ (NADPH氧化酶4阳性)癌症相关成纤维细胞(CAFs)和CXCR4+ (C-X-C基序趋化因子受体4阳性)/GPX4+(谷胱甘肽过氧化物酶4阳性)肿瘤细胞是转移的关键驱动因素。因此,研究人员设计了一种双靶向纳米系统来诱导肿瘤细胞铁下垂并对CAFs进行重编程。该策略将包裹在癌细胞膜内的铁沉诱导剂和加载在癌细胞和CAFs组成的杂交膜上的CXCR4-NOX4抑制剂整合在一起,从而实现双重协同效应:恶性细胞的铁沉根除和诱导CAFs的静止。在原位、肝转移和患者来源的肿瘤异种移植人源化免疫小鼠模型中,这些纳米颗粒显著抑制肿瘤生长,减轻免疫抑制信号,增强抗肿瘤免疫反应,同时保持良好的生物相容性。这些发现强调了同时靶向肿瘤细胞中的铁凋亡和肿瘤微环境中的CAFs重编程以克服结直肠癌肝转移的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


