Reactivity of Alcohol Substrates and Boron-Containing Complexes in C–H Alkylation Enabled by Photoredox, Hydrogen Atom Transfer, and Boronic Acid Catalysis
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-05-21
DOI:10.1021/acs.joc.4c0267010.1021/acs.joc.4c02670
引用次数: 0
Abstract
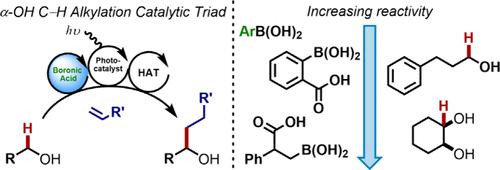
Using boronic acid, photoredox, and HAT catalysis, the relative alkylation reactivity of representative alkyl alcohols was evaluated through competition experiments, revealing higher initial reactivity for diols. Electron-poor arylboronic acid catalysts provide increased reaction efficiency for all substrates, which is attributed to a more dynamic and facile equilibrium between boron-containing species. Furthermore, β-carboxyboronic acids resulted in an additional increase in reaction efficiency, and the results from both catalyst classes were compared using kinetic profiles and a select scope of monoalcohols.
光氧化还原、氢原子转移和硼酸催化下C-H烷基化反应中醇底物和含硼配合物的反应活性
采用硼酸、光氧化还原和HAT催化,通过竞争实验评价了代表性烷基醇的相对烷基化反应活性,揭示了二醇具有较高的初始反应活性。电子贫乏的芳基硼酸催化剂为所有底物提供了更高的反应效率,这归因于含硼物种之间更动态和更容易的平衡。此外,β-羧基硼酸导致了反应效率的额外提高,并且使用动力学曲线和选择的单醇类比较了两类催化剂的结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


