Efficacy and Safety of Low-Dose Spironolactone in Management of Diabetic Kidney Disease in a Real-World Setting
Abstract
Background
Adding spironolactone to renin-angiotensin-aldosterone system (RAAS) blockers has been shown to reduce albuminuria in patients with diabetic kidney disease (DKD). However, the increased risk of hyperkalaemia is a major concern. This study aimed to evaluate the efficacy and safety of low-dose (12.5 mg/day) spironolactone in reducing albuminuria and improving renal function in Iranian adults with DKD.
Methods
This was a pre-post-treatment study of 60 patients with type 2 diabetes, age > 18 years, albuminuria ≥ 30 mg, estimated glomerular filtration rate (eGFR) ≥ 30 mL/min/1.73 m2, and serum potassium level ≤ 5 mEq/L, who were referred to the diabetes clinic of Shariati Hospital, Tehran, Iran. The patients were prescribed spironolactone (12.5 mg) once daily. Changes in urinary albumin excretion (U.Alb), urine albumin-to-creatinine ratio (uACR), blood pressure, serum creatinine (Cr) levels, and serum potassium levels from the baseline over the 12-week intervention period were measured. Statistical significance was set at p < 0.05.
Results
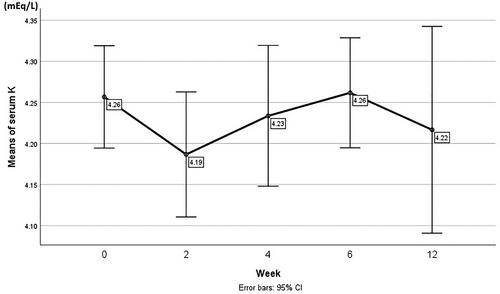
After 12 weeks of taking 12.5 mg/day spironolactone, there was a statistically significant but modest increase in eGFR (p = 0.042), and a statistically significant decrease was observed in both the U.Alb and uACR (p < 0.001). There was a significant reduction in the mean Cr level (p = 0.003). Systolic blood pressure did not decrease significantly (p = 0.079), but diastolic blood pressure decreased significantly (p = 0.007). Changes in serum potassium levels over time were not significant (p = 0.302). The reduction in albuminuria in patients taking only spironolactone and those taking spironolactone with SGLT2i was not significantly different (p = 0.916 and p = 0.948, respectively).
Conclusions
This study found that adding spironolactone to RAAS blockers effectively reduced albuminuria, mildly increased eGFR, and improved renal outcomes in patients with DKD. Additionally, spironolactone significantly reduced albuminuria, regardless of the concurrent use of SGLT2i.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


