Efficient Synthesis of Tetrahydroisoquinoline Alkaloids from Dopamine and Natural Phenolic Acids by Whole-Cell Biocatalysis
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
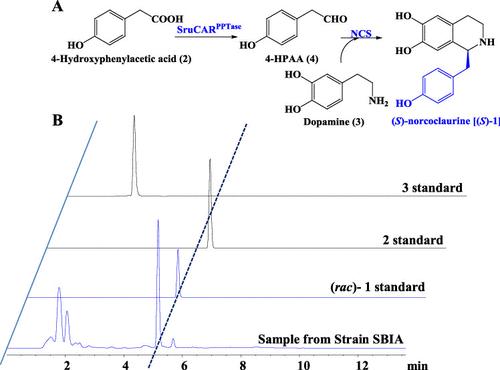
Plant-derived tetrahydroisoquinoline alkaloids (THIQAs) are an important class of natural products for various biological purposes. However, their preparation still relies on phytoextraction, which remains challenging due to low production yields. Notably, biocatalysis has shown the potential to address this challenge. Herein, through overexpressing a carboxylate reductase (CAR), an old yellow enzyme (OYE), and a norcoclaurine synthase (NCS) in Escherichia coli, two whole-cell biocatalytic systems were constructed to synthesize eight THIQAs, including three benzylisoquinoline alkaloids (BIAs) and five phenethylisoquinoline alkaloids (PEIAs), from dopamine and different natural phenolic acids, including phenylacetic and cinnamic acids. The synthesis of BIAs was achieved with excellent enantioselectivity (≥97% ee) and titers of up to 4.34 g L–1. PEIAs could be produced with ee values ranging from 80% to 94%, and most had titers greater than 1 g L–1. Moreover, lignocellulosic hydrolyzate was also used as the substrate to investigate the synthesis of a 1-phenethylisoquinoline scaffold, in which 1.14 g L–1 product was produced in 73.1% yield. This work not only provides a new strategy for the efficient synthesis of diverse THIQAs, but also serves as a reference for the high-value utilization of lignocellulosic biomass in the synthesis of valuable natural products.
多巴胺和天然酚酸全细胞生物催化高效合成四氢异喹啉生物碱
植物源性四氢异喹啉生物碱(THIQAs)是一类重要的天然产物,具有多种生物学用途。然而,它们的制备仍然依赖于植物提取,由于产量低,这仍然具有挑战性。值得注意的是,生物催化已经显示出解决这一挑战的潜力。本研究通过在大肠杆菌中过表达一种羧酸还原酶(CAR)、一种老黄酶(OYE)和一种去甲氯嘌呤合成酶(NCS),构建了两种全细胞生物催化体系,以多巴胺和不同的天然酚酸(苯乙酸和肉桂酸)为原料,合成了8种THIQAs,包括3种苯基异喹啉生物碱(BIAs)和5种苯乙基异喹啉生物碱(PEIAs)。BIAs的合成具有优异的对映体选择性(≥97% ee),滴度高达4.34 g L-1。PEIAs的ee值在80% ~ 94%之间,大多数效价大于1 g L-1。此外,还以木质纤维素水解产物为底物,研究了1-苯乙基异喹啉支架的合成,该支架的L-1产率为73.1%,产率为1.14 g。本研究不仅为高效合成多种THIQAs提供了新的策略,也为木质纤维素生物质在高价值天然产物合成中的高价值利用提供了参考。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


