Advances in the treatment of ANCA-associated vasculitis
IF 32.7
1区 医学
Q1 RHEUMATOLOGY
引用次数: 0
Abstract
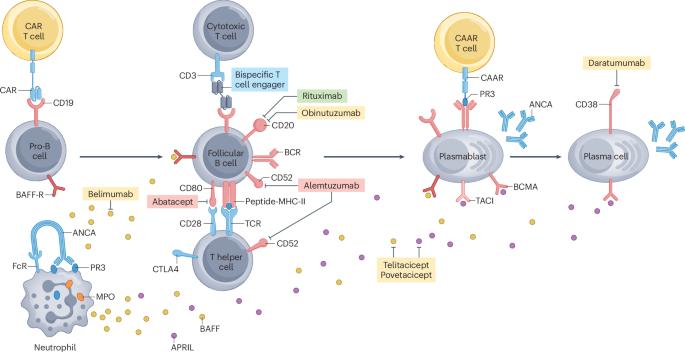
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) consists of a group of small-vessel vasculitides that often present with organ-threatening or life-threatening manifestations. Current immunosuppressive treatments have improved survival and rates of remission, but are not curative, have frequent toxicities, and do not effectively prevent relapse. Clinical trials have established the role of rituximab, an anti-CD20 B cell-depleting monoclonal antibody, in both the remission-induction and maintenance phases of the disease and demonstrated that glucocorticoid doses can be substantially reduced from historical dosing levels without affecting treatment efficacy. Therapies that have the potential to be more effective and safer have become available or are under investigation. Avacopan, an oral C5a receptor antagonist, was approved as an adjunctive treatment for AAV and use of this drug in combination with rituximab or cyclophosphamide and markedly reduced glucocorticoid dosing demonstrated superior efficacy and potentially greater kidney recovery than prior standard of care. Other agents under study for treatment of AAV include next-generation anti-CD20 monoclonal antibodies, anti-CD19 chimeric antigen receptor T cells, novel complement inhibitors and agents that can target fibrosis. Alongside traditional randomized controlled trials with clinical endpoints, experimental medicine studies are focusing on mechanistic endpoints and disease biomarkers. This Review discusses current treatments and the advances in the management of AAV. This Review discusses the advances and challenges of managing antineutrophil cytoplasmic antibody-associated vasculitis. The authors discuss current treatment options, emerging therapies and unmet needs in the management of this disease.

anca相关性血管炎的治疗进展
抗中性粒细胞细胞质抗体(ANCA)相关血管炎(AAV)由一组小血管血管炎组成,通常表现为器官威胁或危及生命的表现。目前的免疫抑制治疗提高了生存率和缓解率,但不能治愈,经常有毒性,不能有效防止复发。临床试验已经确定了利妥昔单抗(一种抗cd20 B细胞消耗单克隆抗体)在疾病的缓解-诱导和维持阶段的作用,并证明糖皮质激素剂量可以从历史剂量水平大幅降低而不影响治疗效果。有可能更有效和更安全的治疗方法已经可用或正在研究中。Avacopan是一种口服C5a受体拮抗剂,已被批准作为AAV的辅助治疗,该药物与利妥昔单抗或环磷酰胺联合使用,并显着减少糖皮质激素剂量,显示出优于先前标准护理的疗效和潜在的更大肾脏恢复。正在研究的其他治疗AAV的药物包括下一代抗cd20单克隆抗体、抗cd19嵌合抗原受体T细胞、新型补体抑制剂和靶向纤维化的药物。除了传统的有临床终点的随机对照试验外,实验医学研究的重点是机械终点和疾病生物标志物。本文综述了目前AAV的治疗方法和治疗进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Rheumatology
医学-风湿病学
CiteScore
29.90
自引率
0.90%
发文量
137
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Rheumatology is part of the Nature Reviews portfolio of journals. The journal scope covers the entire spectrum of rheumatology research. We ensure that our articles are accessible to the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


