Oxidative Iodination of Pyrrolo[2,1-a]isoquinolines with NaI/mCPBA.
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-06-13
Epub Date: 2025-06-04
DOI:10.1021/acs.joc.5c00386
引用次数: 0
Abstract
A mild late-stage modification of pyrrolo[2,1-a]isoquinoline derivatives has been achieved through oxidative iodination with NaI as the iodine source and mCPBA as the oxidant. A series of pyrrolo[2,1-a]isoquinolines has been iodinated readily at ambient temperature in moderate to excellent yield (40→99% yields). Other heteroarenes of great importance such as substituted pyrroles and indoles could also be compatible in this iodination process. It was found that the combination of HI and mCPBA can be alternatively employed for the efficient iodination of heteroarenes efficiently.
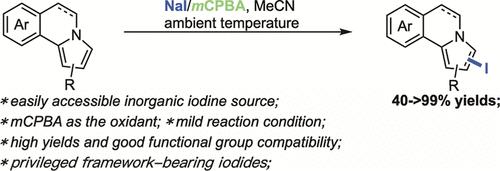
NaI/mCPBA氧化碘化吡咯[2,1-a]异喹啉类化合物。
以NaI为碘源,mCPBA为氧化剂,对吡咯[2,1- A]异喹啉衍生物进行了轻度的后期氧化碘化修饰。一系列吡咯[2,1- A]异喹啉类化合物在常温下容易碘化,产率中至优异(40%→99%)。其他重要的杂芳烃如取代吡咯和吲哚也可以在该碘化过程中相容。结果表明,HI和mCPBA的组合可以有效地替代杂环芳烃的碘化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


