Low-dose radiation by radiopharmaceutical therapy enhances GD2 TRAC-CAR T cell efficacy in localized neuroblastoma
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science Advances
Pub Date : 2025-06-04
引用次数: 0
Abstract
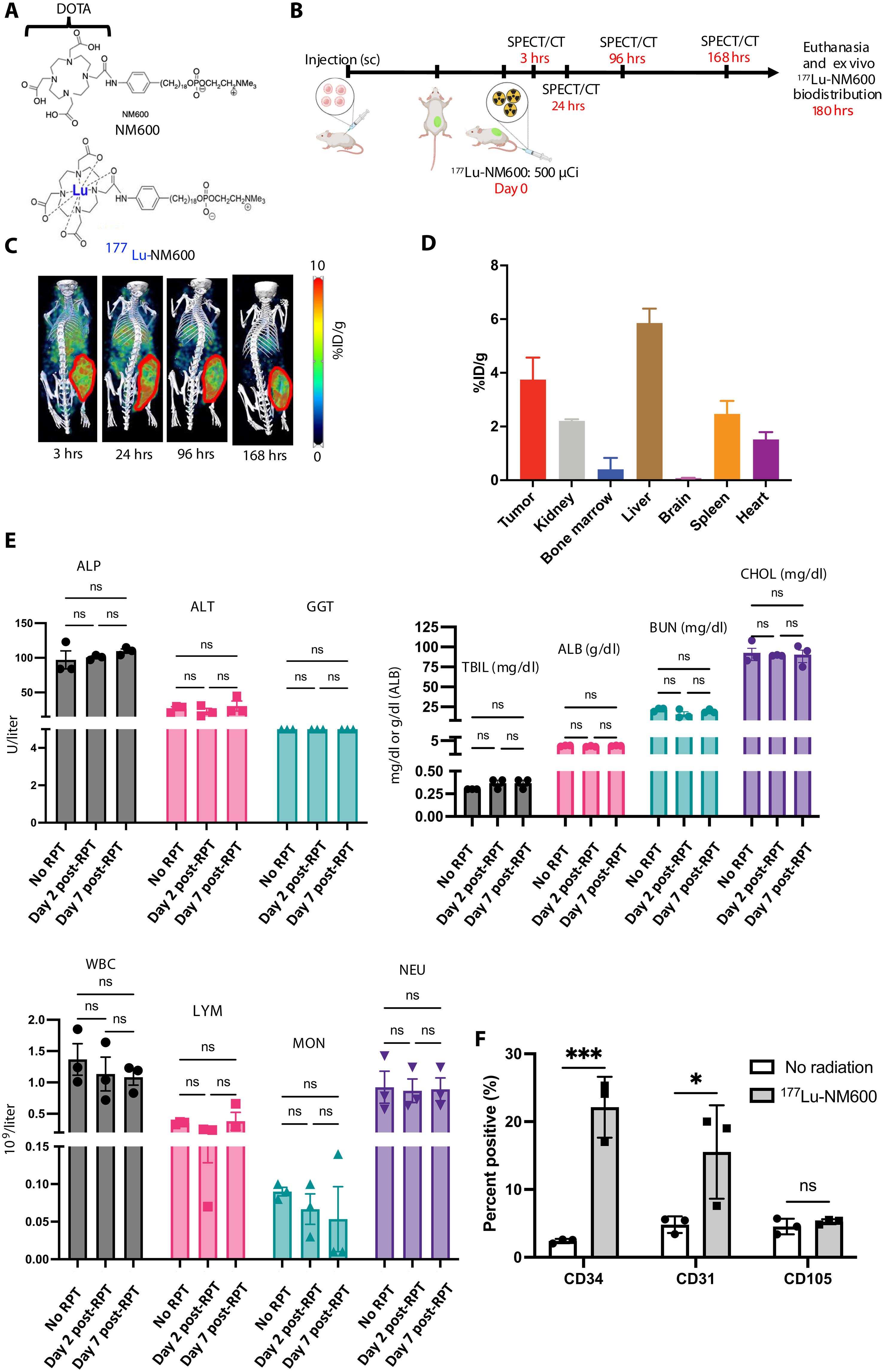
Chimeric antigen receptor (CAR) T cells have limited efficacy against solid tumors including neuroblastoma. Here, we evaluated whether low-dose radiation delivered by radiopharmaceutical therapy (RPT), known to potentiate immune checkpoint inhibitors, can synergize with CRISPR-edited GD2 TRAC-CAR T cells to improve outcomes in neuroblastoma. We found that in the localized model of neuroblastoma, low-dose radiation delivered by 177Lu-NM600, an alkylphosphocholine mimetic RPT agent, followed 9 days later by GD2 TRAC-CAR T cells led to complete tumor regression. Irradiation of neuroblastoma before GD2 TRAC-CAR T cells enhanced the release by CAR T cells of perforin, granzyme B, tumor necrosis factor–α, and interleukin-7 while abrogating transforming growth factor–β1. Low-dose RPT up-regulated the death receptor Fas on neuroblastoma, potentially enabling CAR-independent killing. This suggests that low-dose RPT can enhance suboptimal CAR T cell efficacy against solid tumors. However, optimization of radiation dose and timing may be needed for each patient and RPT agent to account for varied tumor radiosensitivity and dosimetry.
低剂量放射药物治疗提高GD2 track - car - T细胞治疗局部神经母细胞瘤的疗效
嵌合抗原受体(CAR) T细胞对包括神经母细胞瘤在内的实体肿瘤的疗效有限。在这里,我们评估了已知可增强免疫检查点抑制剂的放射性药物治疗(RPT)提供的低剂量辐射是否可以与crispr编辑的GD2 track - car T细胞协同作用,以改善神经母细胞瘤的预后。我们发现,在局部神经母细胞瘤模型中,用177Lu-NM600(一种模拟烷基磷胆碱的RPT药物)低剂量放射,9天后用GD2 track - car - T细胞照射可导致肿瘤完全消退。GD2 track -CAR - T细胞前照射神经母细胞瘤可增强CAR - T细胞对穿孔素、颗粒酶B、肿瘤坏死因子-α和白细胞介素-7的释放,同时消除转化生长因子-β1。低剂量RPT上调神经母细胞瘤的死亡受体Fas,可能实现car非依赖性杀伤。这表明低剂量RPT可以增强CAR - T细胞对实体瘤的治疗效果。然而,考虑到不同的肿瘤放射敏感性和剂量学,可能需要对每个患者和RPT药物的放射剂量和时间进行优化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


