Human aldo-keto reductase (AKR) 1C1 and 1C2 act as coactivators of pregnane X receptor, a master regulator of drug-metabolizing and gluconeogenesis enzymes
IF 2.2
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
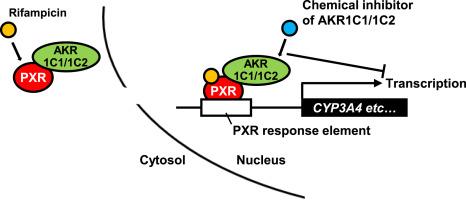
The aldo-keto reductase (AKR) 1C subfamily, comprising AKR1C1-1C4, plays a crucial role in drug metabolism and hormone biosynthesis. Recent studies have identified AKR1C3 as a co-activator of the androgen receptor. This study aimed to investigate whether AKR1Cs function as regulators of the pregnane X receptor (PXR), a member of the nuclear receptor superfamily, which upregulates drug-metabolizing enzymes such as cytochrome P450 (CYP) 3A4. Rifampicin-activated CYP3A4 induction was attenuated by AKR1C1/1C2 knockdown in ShP51 cells (PXR-overexpressing HepG2 cells), HepaRG cells, and HepaSH cells (hepatocytes from humanized liver mice). Co-immunoprecipitation analysis revealed that AKR1Cs interact with PXR. Immunofluorescent staining revealed that AKR1Cs are translocated into the nucleus with PXR by rifampicin in HepaRG cells. These results suggested that AKR1C1/1C2 has an ability to enhance transactivity of PXR. Consistent with the results of knockdown experiments, PXR-mediated CYP3A4 induction was significantly attenuated by treatment with AKR1C1/1C2 inhibitors, diazepam or flunitrazepam, in ShP51, HepaRG, and HepaSH cells. Furthermore, the induction of CYP2B6, CYP2C9, glucose 6-phosphatase, and phosphoenolpyruvate carboxykinase 1, all regulated by PXR, was attenuated by AKR1C1/1C2 inhibitors. Collectively, we demonstrated that AKR1C1/1C2 upregulates PXR transactivation. Clinically used drugs that inhibit AKR1C1/1C2 may suppress PXR-mediated transactivation of genes encoding drug-metabolizing and gluconeogenesis enzymes.
人醛酮还原酶(AKR) 1C1和1C2是孕激素X受体的共激活因子,是药物代谢和糖异生酶的主要调节因子
醛酮还原酶(AKR) 1C亚家族包括AKR1C1-1C4,在药物代谢和激素生物合成中起重要作用。最近的研究已经确定AKR1C3是雄激素受体的共激活剂。本研究旨在研究AKR1Cs是否作为妊娠X受体(PXR)的调节因子,PXR是核受体超家族的成员,可上调细胞色素P450 (CYP) 3A4等药物代谢酶。在ShP51细胞(pxr过表达HepG2细胞)、HepaRG细胞和HepaSH细胞(人源化肝小鼠的肝细胞)中,akr11.1 / 1c2敲低可减弱利福平激活的CYP3A4诱导。共免疫沉淀分析显示akr1c与PXR相互作用。免疫荧光染色显示,在HepaRG细胞中,akr1c通过利福平与PXR一起易位到细胞核中。这些结果表明,akr11.1 / 1c2具有增强PXR交互活性的能力。与敲低实验结果一致,在ShP51、HepaRG和HepaSH细胞中,使用akr11.1 / 1c2抑制剂地西泮或氟硝西泮治疗后,pxr介导的CYP3A4诱导显著减弱。此外,由PXR调控的CYP2B6、CYP2C9、葡萄糖6-磷酸酶和磷酸烯醇丙酮酸羧激酶1的诱导作用被akr11.1 / 1c2抑制剂减弱。总的来说,我们证明了akr11.1 / 1c2上调PXR的转录激活。临床使用的抑制akr11.1 / 1c2的药物可能会抑制pxr介导的药物代谢和糖异生酶编码基因的转激活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.80
自引率
9.50%
发文量
50
审稿时长
69 days
期刊介绍:
DMPK publishes original and innovative scientific papers that address topics broadly related to xenobiotics. The term xenobiotic includes medicinal as well as environmental and agricultural chemicals and macromolecules. The journal is organized into sections as follows:
- Drug metabolism / Biotransformation
- Pharmacokinetics and pharmacodynamics
- Toxicokinetics and toxicodynamics
- Drug-drug interaction / Drug-food interaction
- Mechanism of drug absorption and disposition (including transporter)
- Drug delivery system
- Clinical pharmacy and pharmacology
- Analytical method
- Factors affecting drug metabolism and transport
- Expression of genes for drug-metabolizing enzymes and transporters
- Pharmacogenetics and pharmacogenomics
- Pharmacoepidemiology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


