Unraveling the Surface Termination and Evolution of Surface States for Electrocatalyst PtSn4 in Alkaline HER
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
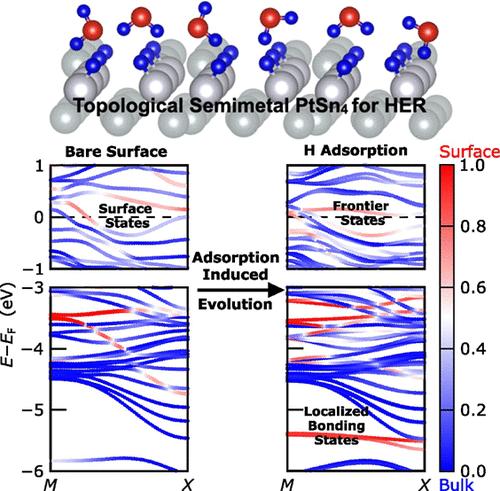
Semimetal PtSn4 has been experimentally demonstrated as a promising topological electrocatalyst for the hydrogen evolution reaction (HER) under both acidic and alkaline conditions. While two possible mechanisms have been proposed to explain its activity, the role of its surface states in HER remains unclear. It is indeed in question how the surface states of this alloy evolve as HER proceeds. In this study, we investigate the surface termination that sustains conducting surface states on PtSn4, and we track their evolution during HER catalysis. We show that a reconstructed surface with a Sn-poor termination reproduces the scanning tunneling microscopy pattern observed in experiments and sustains a conducting surface. Through phase diagram and geometric structure analysis, we outline the HER profile following the Volmer–Heyrovsky mechanism. As hydrogen atoms adsorb onto the surface, the structure undergoes further reconstruction to an equilibrium phase with a coverage of two hydrides per unit cell. Meanwhile, the surface electronic bands evolve in response to interactions with the adsorbed hydrogen atoms. A hybridization diagram is further proposed for understanding the surface state evolution based on wave function and chemical bonding analyses. While the Pt atoms serve as conventional sites for hydrogen binding, the surface states of PtSn4 are essential for stabilizing the hydrogen antibonding states via in-phase electronic interactions with the Sn components. This stabilization results in frontier surface bands that are responsible for driving the HER catalysis. Our findings provide a detailed description for the direct involvement of surface states on PtSn4 when employed as a catalyst for HER.
电催化剂PtSn4在碱性HER中的表面终止和表面态演化
半金属PtSn4已被实验证明是一种在酸性和碱性条件下很有前途的析氢反应(HER)拓扑电催化剂。虽然已经提出了两种可能的机制来解释其活性,但其表面状态在HER中的作用仍不清楚。随着HER的进行,这种合金的表面状态如何演变确实是个问题。在这项研究中,我们研究了维持PtSn4导电表面态的表面终止,并跟踪了它们在HER催化过程中的演变。我们发现,具有贫锡末端的重建表面再现了实验中观察到的扫描隧道显微镜模式,并维持了导电表面。通过相图和几何结构分析,我们勾勒出了遵循Volmer-Heyrovsky机制的HER轮廓。当氢原子吸附到表面时,结构进一步重建到平衡相,每个单元电池覆盖两个氢化物。同时,表面电子带随着与吸附氢原子的相互作用而演化。进一步提出了一种基于波函数和化学键分析的杂化图来理解表面态的演变。虽然Pt原子是氢结合的传统位点,但PtSn4的表面状态对于通过与Sn组分的相电子相互作用来稳定氢反键状态至关重要。这种稳定化产生了边界表面带,负责驱动HER催化。我们的发现为PtSn4作为HER催化剂时表面状态的直接参与提供了详细的描述。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


