Precise multiphase hydrogel engineering of miniaturized 3D cancer architectures via computationally informed microfluidics
IF 17.5
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Understanding cancer biology and therapy responses requires accurate in vitro models that reflect tumor complexity. This work presents a multiphase microfluidic biofabrication approach for creating self-standing three-dimensional (3D) tumor models within hydrogel microfiber boundaries. A single framework enabled the fast generation of different in vitro cellular configurations, including discrete spheroids in size-limited liquid pockets and continuous multicellular fiberoids. These constructs incorporate key tumor features, including solid stress and microenvironmental interactions, which contribute to a more physiologically relevant replication of tumor responses. Computational simulations were used to fine-tune the biofabrication process, predicting fiber shapes and reducing costs associated with experimental iterations. In vitro tests demonstrated drug responsiveness in all configurations, with greatly enhanced manipulation of soft 3D cell structures. The fiberoid models further emulated intercellular dynamics herein explored in the glioblastoma-astrocyte context, expanding the versatility of our technology for cancer research, as a promising tool for drug discovery and precision-medicine strategies.
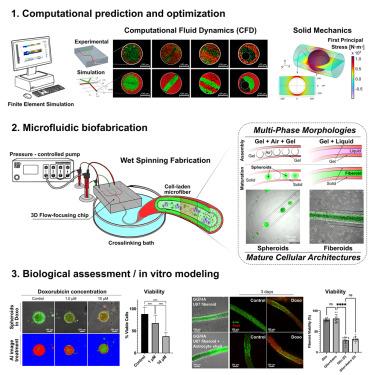

微型三维癌症结构的精确多相水凝胶工程
了解癌症生物学和治疗反应需要准确的体外模型来反映肿瘤的复杂性。这项工作提出了一种多相微流控生物制造方法,用于在水凝胶微纤维边界内创建独立的三维(3D)肿瘤模型。一个单一的框架能够快速生成不同的体外细胞结构,包括尺寸有限的液体口袋中的离散球体和连续的多细胞纤维体。这些结构结合了肿瘤的关键特征,包括固体压力和微环境相互作用,这有助于更生理相关的肿瘤反应复制。计算模拟用于微调生物制造过程,预测纤维形状并降低与实验迭代相关的成本。体外试验表明,在所有配置的药物反应性,大大增强了软三维细胞结构的操作。纤维样模型进一步模拟了胶质母细胞瘤-星形胶质细胞背景下的细胞间动力学,扩大了我们癌症研究技术的多功能性,作为药物发现和精确医学策略的有前途的工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Matter
MATERIALS SCIENCE, MULTIDISCIPLINARY-
CiteScore
26.30
自引率
2.60%
发文量
367
期刊介绍:
Matter, a monthly journal affiliated with Cell, spans the broad field of materials science from nano to macro levels,covering fundamentals to applications. Embracing groundbreaking technologies,it includes full-length research articles,reviews, perspectives,previews, opinions, personnel stories, and general editorial content.
Matter aims to be the primary resource for researchers in academia and industry, inspiring the next generation of materials scientists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


