Formation of an expanding memory representation in the hippocampus
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
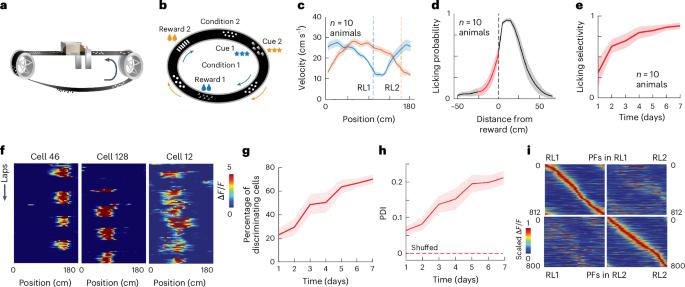
How brain networks connected by labile synapses store new information without catastrophically overwriting previous memories remains poorly understood. To examine this, we tracked the same population of hippocampal CA1 place cells (PCs) as mice learned a task for 7 days. We found evidence of memory formation as both the number of PCs maintaining a stable place field and the stability of individual PCs progressively increased across the week until most of the representation was composed of long-term stable PCs. The stable PCs disproportionately represented task-related learned information, were retrieved earlier within a behavioral session and showed a strong correlation with behavioral performance. Both the initial formation of PCs and their retrieval on subsequent days were accompanied by prominent signs of behavioral timescale synaptic plasticity (BTSP), suggesting that even stable PCs were re-formed by synaptic plasticity each session. Further experimental evidence supported by a cascade-type state model indicates that CA1 PCs increase their stability each day they are active, eventually forming a highly stable population. The results suggest that CA1 memory is implemented by an increase in the likelihood of new neuron-specific synaptic plasticity, as opposed to extensive long-term synaptic weight stabilization. Multiday imaging of CA1 neurons during learning reveals that the representation stabilizes as the number of readily retrievable, information-rich and stable place cells increases and suggests novel mechanisms of hippocampal memory formation.

海马体中扩展记忆表征的形成
由不稳定的突触连接的大脑网络如何存储新信息,而不会灾难性地覆盖以前的记忆,人们仍然知之甚少。为了验证这一点,我们在小鼠学习一项任务的7天时间里追踪了相同数量的海马CA1位置细胞(PCs)。我们发现了记忆形成的证据,因为保持稳定位置场的pc数量和单个pc的稳定性在一周内逐渐增加,直到大部分表征由长期稳定的pc组成。稳定的个人电脑不成比例地代表了与任务相关的学习信息,在行为会话中较早被检索,并显示出与行为表现的强烈相关性。pc的初始形成和随后几天的恢复都伴随着明显的行为时间尺度突触可塑性(BTSP)迹象,这表明即使是稳定的pc也会在每次会话中被突触可塑性重新形成。由级联型状态模型支持的进一步实验证据表明,CA1 pc在活跃的每一天都会增加它们的稳定性,最终形成一个高度稳定的种群。结果表明,CA1记忆是通过增加新的神经元特异性突触可塑性的可能性来实现的,而不是广泛的长期突触重量稳定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


