Heparan Sulfate-Based Neoproteoglycan for Targeted Lysosomal Degradation of Amyloid-β
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Targeted lysosomal degradation of proteins (LDP) represents a promising strategy for clearing unwanted toxic extracellular and secreted proteins. Yet, significant challenges persist, including identifying potential ligands for these proteins and lysosome-driving probes capable of facilitating their internalization and degradation through receptor-mediated endocytosis. Herein, we show that synthetic neoproteoglycan probes stably anchor to the cell membrane, facilitate the internalization of amyloid-β (Aβ) peptide into the lysosomal compartment, and mediate the programmed death of Aβ. We have identified sulfated oligo l-idose tetrasaccharide (ID49) and heparan sulfate hexasaccharides (HH26S) as potential ligands for Aβ1–42 peptide. When these molecules are expressed on the peptide-based fluorescent neoproteoglycan backbone, PG@HH26S persists on the cell membrane and facilitates Aβ1–42 endocytosis to the lysosomal compartment and subsequent targeted degradation of Aβ1–42. Overall, neoproteoglycans open a new avenue to generate LDP for degrading HS-binding proteins, including growth factors, morphogens, and toxic secreted proteins.
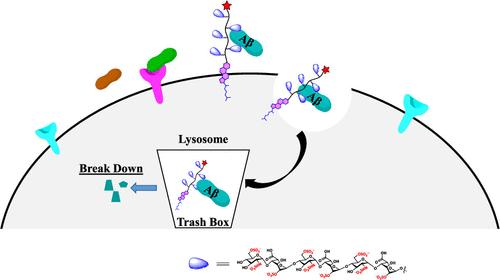
以硫酸肝素为基础的新蛋白多糖用于靶向溶酶体降解淀粉样蛋白-β
靶向溶酶体降解蛋白(LDP)是清除不需要的有毒细胞外和分泌蛋白的一种有前途的策略。然而,重大的挑战仍然存在,包括确定这些蛋白质的潜在配体和溶酶体驱动探针,这些探针能够通过受体介导的内吞作用促进它们的内化和降解。本研究表明,合成的新蛋白多糖探针可以稳定地锚定在细胞膜上,促进淀粉样蛋白-β (Aβ)肽内化到溶酶体腔室,并介导Aβ的程序性死亡。我们已经鉴定出硫酸寡糖(ID49)和硫酸肝素六糖(HH26S)作为a - β1 - 42肽的潜在配体。当这些分子在基于肽的荧光新蛋白聚糖主链上表达时,PG@HH26S在细胞膜上持续存在,促进Aβ1-42内吞到溶酶体腔室,随后靶向降解Aβ1-42。总的来说,新蛋白聚糖为生成降解hs结合蛋白(包括生长因子、形态因子和有毒分泌蛋白)的LDP开辟了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


