Carbon nanotubes for boosting the performance of aqueous zinc-ion battery based on vanadium hexacyanoferrate cathode materials: The impact of its structure and N-doping
IF 5.6
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
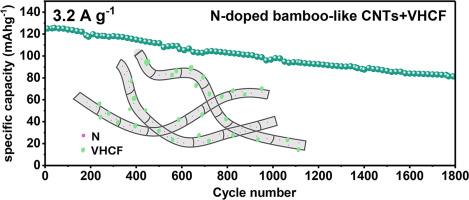
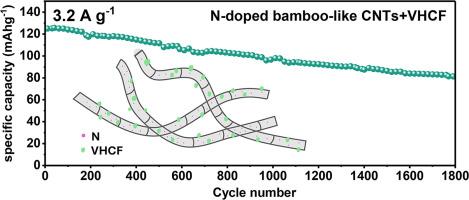
Vanadium hexacyanoferrate (VHCF) have emerged as promising cathode materials for aqueous zinc-ion batteries (AZIBs) due to their open framework and tunable redox properties. However, their low electrical conductivity and limited cycling stability hinder practical applications. To address these challenges, this study integrates VHCF with various carbon nanotubes (parallel nanotubes (P-CNTs), fishbone-like nanotubes (FB-CNTs), and N-doped bamboo-like nanotubes (N/B-CNTs)) via a one-step co-precipitation method. XRD, XPS, and FTIR results confirmed the successful synthesis of VHCF/CNT composites with negligible change of the VHCF structure and composition. Additionally, the incorporation of N-doped bamboo-like CNTs achieved a 3-5-fold increase in specific surface up to 198.2 m2 g-1. Battery evaluations demonstrated significant enhancement of capacity and cycling stability after the incorporation of CNTs: N/B-CNTs delivered the highest specific capacity (∼125 mAh g-1 at 3.2 A g-1), superior rate capability (85.4% capacity retention from 0.2 to 3.2 A g-1), and exceptional cycling stability (81.4 mAh g-1 after 1800 cycles, 65.1% retention), outperforming pristine VHCF (39.5%), P-CNTs (56.9%), and FB-CNTs (54.8%). Electrochemical analysis resolved the role of CNT structures and nitrogen doping in during battery tests. It reveals that cross-linked CNTs provided additional mechanical support and subsequently promoted charge transfer kinetics. Moreover, the highly dispersed VHCF on CNTs and improved surface area enhanced electron exchange process and Zn2+ storage. The superior performance of N/B-CNTs is attributed to the enhanced electronic conductivity from nitrogen doping and the unique bamboo-like structure, which further facilitates ion diffusion and provides robust mechanical stability.
碳纳米管提高六氰高铁钒正极材料锌离子电池性能:结构和n掺杂的影响
六氰高铁钒(VHCF)由于其开放的结构和可调的氧化还原性能而成为很有前途的水性锌离子电池(AZIBs)正极材料。然而,它们的低导电性和有限的循环稳定性阻碍了实际应用。为了解决这些挑战,本研究通过一步共沉淀法将VHCF与各种碳纳米管(平行纳米管(P-CNTs),鱼骨状纳米管(FB-CNTs)和N掺杂竹状纳米管(N/B-CNTs))集成在一起。XRD, XPS和FTIR结果证实了VHCF/CNT复合材料的成功合成,VHCF的结构和组成变化可以忽略不计。此外,掺入n掺杂的竹状碳纳米管使比表面积增加了3-5倍,达到198.2 m2 g-1。电池评估表明,加入CNTs后,电池容量和循环稳定性显著增强:N/B-CNTs提供最高比容量(在3.2 A g-1时约125 mAh g-1),优越的倍率能力(从0.2 A g-1到3.2 A g-1时容量保持85.4%)和卓越的循环稳定性(1800次循环后81.4 mAh g-1,保持65.1%),优于原始VHCF(39.5%)、P-CNTs(56.9%)和FB-CNTs(54.8%)。电化学分析解决了碳纳米管结构和氮掺杂在电池测试中的作用。这表明交联的碳纳米管提供了额外的机械支持,并随后促进了电荷转移动力学。此外,碳纳米管上高度分散的VHCF和改善的表面积增强了电子交换过程和Zn2+的储存。N/B-CNTs的优异性能归功于氮掺杂增强的电子导电性和独特的竹状结构,这进一步促进了离子的扩散,并提供了强大的机械稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


