Concentration-Dependent Thermodynamics and Kinetics in Lithium-Metal Battery Electrolytes: Implications for Coulombic Efficiency
IF 18.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Lithium (Li)-metal batteries (LMBs) are promising for high-energy applications but are hindered by dendrite formation and unstable interphases. This study investigated Li plating thermodynamics and kinetics as descriptors governing Coulombic efficiency (CE), focusing on lithium bis(fluorosulfonyl)imide in fluoroethylene carbonate and 1,2-dimethoxyethane electrolytes. First, upshifts in Li+/Li potential (ELi+/Li vs Me10Fc), via low donor number solvents and high salt concentrations, correlated with increased CEs. Second, reaction (ΔSLi+Li) and configurational entropy (SC), obtained via Seebeck coefficient and heat capacity measurements, respectively, did not correlate with CE and formed a minority contribution to Li+/Li reaction free energy, revealing the primarily enthalpic origin of the ELi+/Li correlation. Finally, kinetic analyses to examine the balance of exchange current density (j0) and diffusion (DLi) showing lower DLi/j0 led to higher CE. These CE correlations highlight the combined effects of thermodynamics and kinetics on Li reversibility, informing rational strategies for enhanced performance of LMBs.
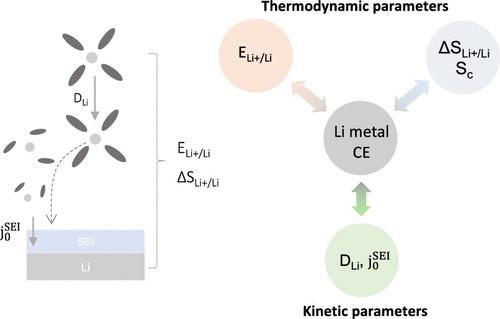
锂金属电池电解质的浓度依赖性热力学和动力学:对库仑效率的影响
锂(Li)-金属电池(lmb)在高能应用方面很有前景,但由于枝晶的形成和界面不稳定而受到阻碍。本研究以二氟磺酰亚胺锂在氟碳酸乙烯和1,2-二甲氧基乙烷电解质中的镀锂热力学和动力学为研究对象,研究了其作为库仑效率(CE)描述子的镀锂热力学和动力学。首先,通过低供体数溶剂和高盐浓度,Li+/Li电位(ELi+/Li vs Me10Fc)的上升与ce的增加相关。其次,分别通过Seebeck系数和热容测量得到的反应(ΔSLi+Li)和构型熵(SC)与CE不相关,对Li+/Li反应自由能的贡献很小,揭示了ELi+/Li相关的主要焓源。最后,动力学分析检验了交换电流密度(j0)和扩散(DLi)的平衡,表明DLi/j0越低,CE越高。这些CE相关性突出了热力学和动力学对Li可逆性的综合影响,为提高lmb性能提供了合理的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


