Site‐Selective CH Amination of Lupane‐Type Triterpenoids
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A synthetic protocol for rhodium‐catalyzed, site‐selective CH amination of the betulin scaffold has been developed. Under catalytic conditions, betulin‐derived 28‐O‐sulfamate ester undergoes intramolecular CH amination to afford 1,2,3‐oxathiazinane‐2,2‐dione‐fused lupane triterpenoids with a C16/C22 selectivity ratio 9:1. Introduction of a C16‐substituent enables a second sequential CH amination, which occurs with C22 selectivity. Betulin‐derived oxathiazinanes can be cleaved and the ring‐opened products provide straightforward access to 16‐amino‐ and 16‐azido‐betulin, 16‐amino‐betulinic, and 16‐amino‐betulonic acids. Betulin analogs with selectively introduced amine and azide functionalities are versatile building blocks for further medicinal chemistry applications in semisynthetic triterpenoid series.
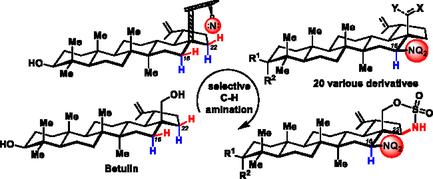
Lupane型三萜的位点选择性C - H胺化
一个合成方案铑催化,位点选择性的C - H胺化白桦脂支架已经开发。在催化条件下,桦木素衍生的28 - O -氨基磺酸酯发生分子内C - H胺化反应,得到1,2,3 -恶噻嗪- 2,2 -二酮融合的狼烷三萜,C16/C22选择性比为9:1。引入C16取代基可以实现C22选择性的第二次连续C - H胺化。桦木素衍生的恶噻嗪烷可以被切割,打开环的产物可以直接接触到16 -氨基和16 -叠氮-桦木素、16 -氨基-桦木酸和16 -氨基-桦木酸。具有选择性引入胺和叠氮化物功能的白桦林类似物是半合成三萜系列进一步药物化学应用的通用构建块。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


