Mating strategy does not affect the diversification of abdominal chemicals in Heliconiini butterflies
Abstract
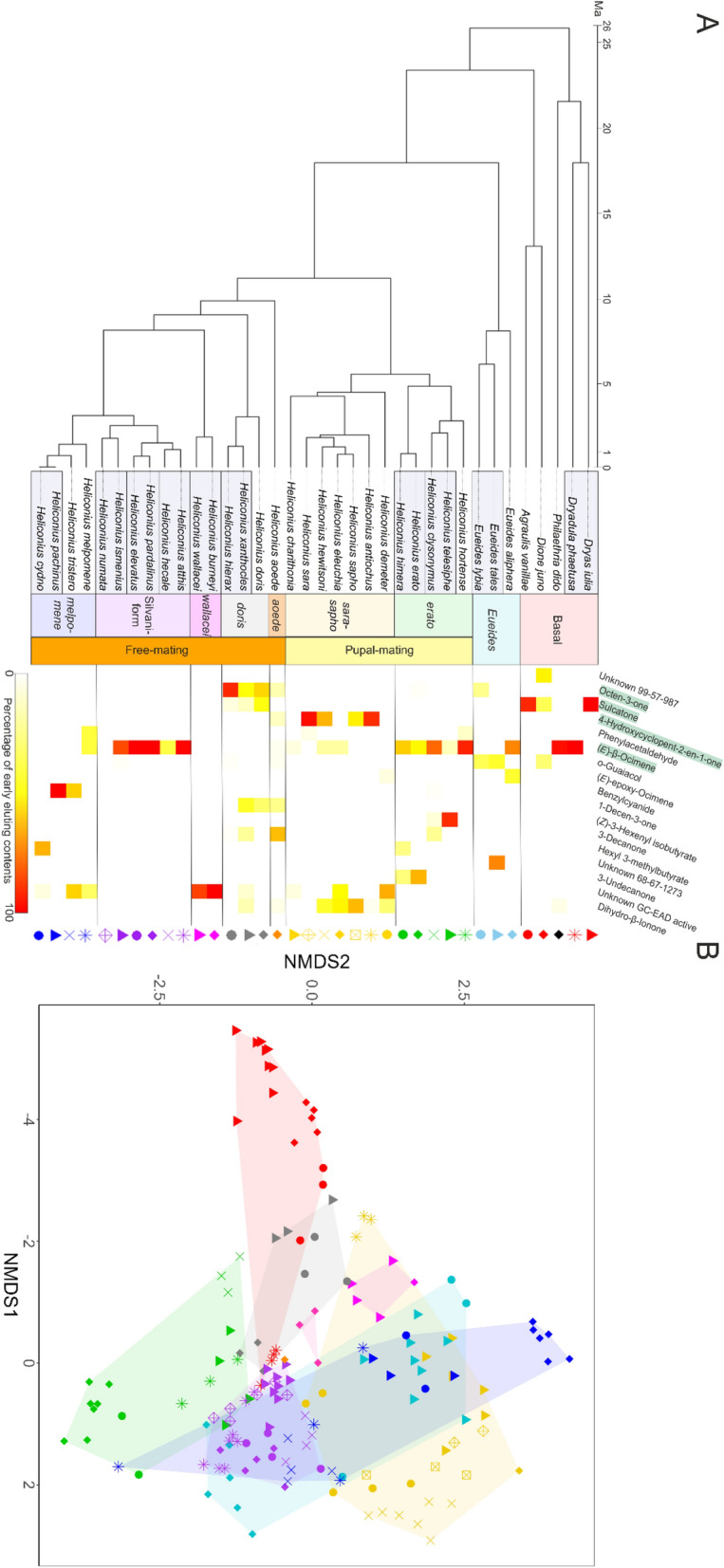
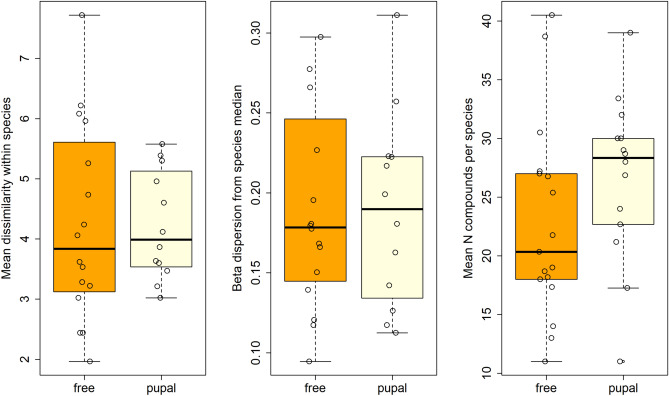
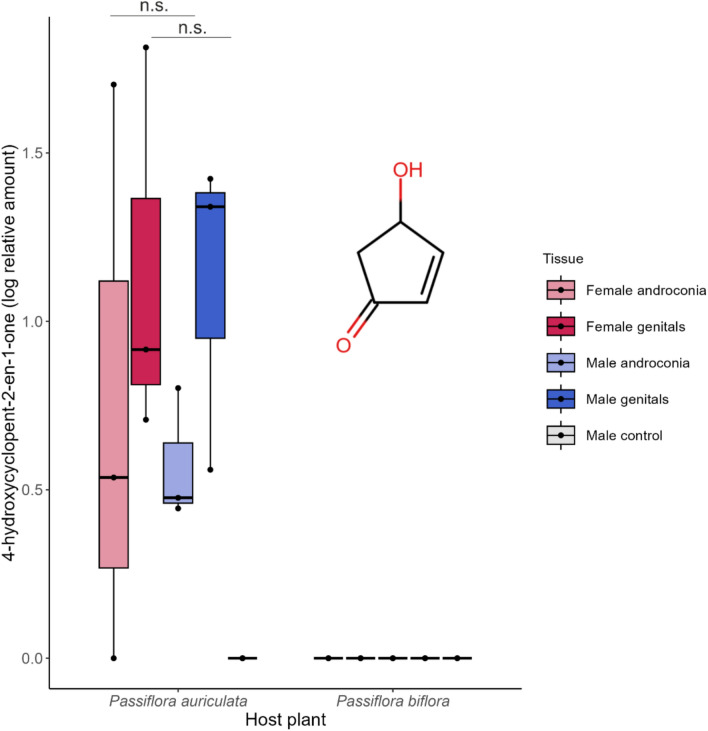
Antiaphrodisiacs are chemical bouquets physically delivered from male to female individuals upon copulation which discourage further mating and reduce sperm competition by rendering the female less attractive. Since antiaphrodisiacs may not offer an honest signal of female receptivity, in polyandrous species they may undergo faster diversification resulting from sexual conflict. The Heliconiini tribe of butterflies includes a polyandrous (free-mating) and a monandrous (pupal-mating) clade, both known to produce diverse antiaphrodisiac mixtures as part of their abdominal blends. Using multivariate phylogenetic comparative methods, we analyzed the genital blends of 36 Heliconiini species to test the hypothesis that blend diversity results from male-male competition in polyandry. We found no evidence for shifts in blend diversification rate corresponding to changes in mating strategy, implying male-male competition may have a weaker effect on pheromone diversification in this group than previously thought. The genital blends of most species are dominated by one of four highly volatile compounds; (E)-β-ocimene, octen-3-one, sulcatone and 4-hydroxycyclopent-2-en-1-one. Based on the function of (E)-β-ocimene as the behaviourally active antiaphrodisiac in H. melpomene, we propose a similar role in other species for the other volatiles. We test this hypothesis by investigating 4-hydroxycyclopent-2-en-1-one occurrence in Heliconius sara. While we detect no sex-based differences on its presence, we find the compound is undetectable when larvae are not fed their preferred host plant, providing an intriguing potential link between host plant and reproductive cues. This in turn shows that captive-bred samples do not always provide realistic results and this awareness is important for future experiments.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


