Mechanism of uranium retention via reductive biotite under the aerobic conditions: Role of structural ferrous iron
IF 5.8
2区 地球科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
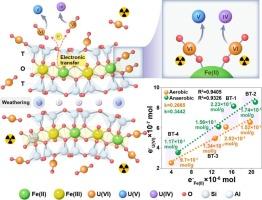
A comprehensive understanding of the environmental fate of uranium necessitates a thorough understanding of the sequestration mechanism of uranium on typical rock-forming minerals. In this work, the sorption behavior and mechanism of uranium on four kinds of biotite with different iron contents were investigated, and the role of structural ferrous iron in uranium sorption was determined with spectroscopic techniques. The results indicated that the interactions of U(VI) with biotite are governed primarily by redox reactions and surface complexation. The reduction in the U(VI) is induced by structural ferrous iron, which accounts for approximately half of the iron content, and an increased iron content in biotite can augment the number of binding and reduction sites available for U(VI). Biotite weathering facilitated U(VI) reduction to UO2 or hyperstoichiometric uranium oxides, and the amounts of electrons transferred between ferrous iron and the absorbed U(VI) exhibited a linear relationship, which highlights the pivotal role of ferrous iron in uranium sorption. Uranium sorption in anaerobic conditions exceeds aerobic conditions due to improved electron transport and reduction efficiency. These insights are essential for understanding the mechanisms behind uranium retention in biotite-rich granitic terrains and offer valuable perspectives on potential strategies for remediating uranium-contaminated sites.
好氧条件下还原性黑云母对铀的保留机理:结构亚铁的作用
要全面了解铀的环境命运,就必须彻底了解铀在典型造岩矿物上的封存机制。本文研究了四种不同含铁量的黑云母对铀的吸附行为和机理,并用光谱技术测定了结构铁在铀吸附中的作用。结果表明,U(VI)与黑云母的相互作用主要由氧化还原反应和表面络合作用控制。U(VI)的还原是由约占铁含量一半的结构亚铁引起的,黑云母中铁含量的增加可以增加U(VI)的结合和还原位点的数量。黑云母风化作用有利于U(VI)还原为UO2或高化学量铀氧化物,铁亚铁与吸附U(VI)之间的电子转移量呈线性关系,凸显了铁亚铁在铀吸附中的关键作用。由于电子传递和还原效率的提高,厌氧条件下铀的吸附优于好氧条件。这些见解对于理解富黑云母花岗岩地形中铀保留的机制至关重要,并为修复铀污染场地的潜在策略提供了有价值的观点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Clay Science
地学-矿物学
CiteScore
10.30
自引率
10.70%
发文量
289
审稿时长
39 days
期刊介绍:
Applied Clay Science aims to be an international journal attracting high quality scientific papers on clays and clay minerals, including research papers, reviews, and technical notes. The journal covers typical subjects of Fundamental and Applied Clay Science such as:
• Synthesis and purification
• Structural, crystallographic and mineralogical properties of clays and clay minerals
• Thermal properties of clays and clay minerals
• Physico-chemical properties including i) surface and interface properties; ii) thermodynamic properties; iii) mechanical properties
• Interaction with water, with polar and apolar molecules
• Colloidal properties and rheology
• Adsorption, Intercalation, Ionic exchange
• Genesis and deposits of clay minerals
• Geology and geochemistry of clays
• Modification of clays and clay minerals properties by thermal and physical treatments
• Modification by chemical treatments with organic and inorganic molecules(organoclays, pillared clays)
• Modification by biological microorganisms. etc...
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


