Directional generation of singlet oxygen (1O2) for efficient antibiotic degradation via Cu-Co hierarchical activation of molecular oxygen
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
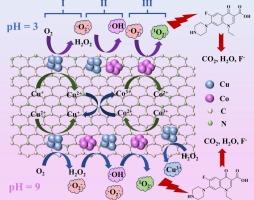
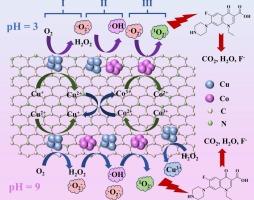
Singlet oxygen (1O2) offers unique advantages for contaminant degradation owing to its high oxidative selectivity, long half-life, and pH-independent reactivity. However, the spin-forbidden transition between molecular oxygen (triplet state, 3O2) and 1O2 severely limits their interconversion under energy-free conditions. Herein, we innovatively prepared a nitrogen-doped carbon-coated copper-cobalt alloy catalyst (CuCo@NC) that hierarchically activated molecular oxygen into 1O2. The optimized CuCo@NC600 demonstrated exceptional performance: achieving 96.79 %-98.91 % Norfloxacin (NOR) removal and ∼70 % mineralization within 40 min across a broad pH range of 3–11 under conditions of 1.0 g/L catalyst and 20 mg/L NOR. The catalyst exhibited remarkable versatility, degrading diverse organic pollutants (rhodamine B, tetracycline hydrochloride, etc.) with > 90 % efficiency and strong anti-interference capability against common ions and organic matter. No significant performance degradation was observed in both real wastewater and surface water systems. Radical quenching experiments confirmed 1O2 as the dominant reactive species generated through hierarchically activating O2 by Cu-Co collaboration: molecular oxygen is initially activated at Cu sites, generating •O2– and •OH, which are then captured and converted to 1O2 at Co sites. The preserved catalytic stability (>70 % efficiency after 3 cycles) originated from the protective carbon matrix and self-compensating Cu+/Cu2+–Co2+/Co3+ valence transitions. LC-MS-identified intermediates revealed three detoxification pathways. This work provided an energy-efficient and environmentally friendly strategy for 1O2-dominated advanced oxidation processes, demonstrating significant potential for antibiotic-polluted water remediation.
定向生成单线态氧(1O2),通过Cu-Co分子氧的分层活化有效降解抗生素
单线态氧(1O2)由于其高氧化选择性、长半衰期和不依赖于ph的反应性,为污染物降解提供了独特的优势。然而,分子氧(三重态,3O2)和1O2之间的自旋禁止跃迁严重限制了它们在无能条件下的相互转化。在此,我们创新地制备了一种氮掺杂碳包覆铜钴合金催化剂(CuCo@NC),该催化剂将分子氧分层活化为1O2。优化后的CuCo@NC600表现出优异的性能:在1.0 g/L催化剂和20 mg/L NOR的条件下,在3-11的广泛pH范围内,在40 min内实现了96.79 %-98.91 %的诺氟沙星(NOR)去除率和 ~ 70 %的矿化。该催化剂具有良好的多功能性,可降解多种有机污染物(罗丹明B、盐酸四环素等),效率为 >; 90 %,对普通离子和有机物具有较强的抗干扰能力。在实际废水和地表水系统中均未观察到明显的性能下降。自由基猝灭实验证实,通过Cu-Co协同作用分层活化O2产生的优势活性物质是1O2:分子氧最初在Cu位点被激活,生成•O2 -和•OH,然后在Co位点被捕获并转化为1O2。3次循环后的催化稳定性(>70 %)源于保护碳基质和自补偿Cu+/Cu2+ -Co2 +/Co3+价态跃迁。lc - ms鉴定的中间体揭示了三种解毒途径。这项工作为以二氧化氧为主的高级氧化工艺提供了一种节能环保的策略,显示了抗生素污染水修复的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


