Comparative development and evaluation of Fe–N–C electrocatalysts for the oxygen reduction reaction: The effect of pyrolysis and iron-bipyridine structures
IF 13.8
引用次数: 0
Abstract
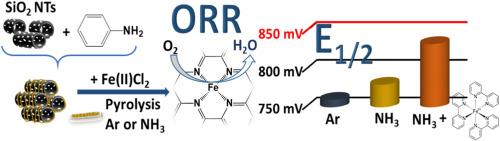
Proton exchange membrane fuel cells (PEMFCs) constitute a promising avenue for environmentally friendly power generation. However, the reliance on unsustainable platinum-based electrocatalysts used at the electrodes poses challenges to the commercial viability of PEMFCs. Non-platinum group metal (non-PGM) alternatives, like nitrogen-coordinated transition metals in atomic dispersion (M–N–C catalysts), show significant potential. This work presents a comparative study of two distinct sets of Fe–N–C materials, prepared by pyrolyzing hybrid composites of polyaniline (PANI) and iron (II) chloride on a hard template. One set uses bipyridine (BPy) as an additional nitrogen source and iron ligand, offering an innovative approach. The findings reveal that the choice of pyrolysis temperature and atmosphere influences the catalyst properties. The use of ammonia in pyrolysis emerges as a crucial parameter for promoting atomic dispersion of iron, as well as increasing surface area and porosity. The optimal catalyst, prepared using BPy and ammonia, exhibits a half-wave potential of 0.834 V in 0.5 M H2SO4 (catalyst loading of 0.6 mg cm−2), a mass activity exceeding 3 A g−1 and high stability in acidic electrolyte, positioning it as a promising non-PGM structure in the field.
Fe-N-C氧还原反应电催化剂的比较开发与评价:热解和铁联吡啶结构的影响
质子交换膜燃料电池(pemfc)是一种很有前途的环保发电技术。然而,依靠不可持续的铂基电催化剂用于电极,对pemfc的商业可行性提出了挑战。非铂族金属(non-PGM)替代品,如氮配位原子分散过渡金属(M-N-C催化剂),显示出巨大的潜力。本文介绍了两种不同的Fe-N-C材料的比较研究,这些材料是通过在硬模板上热解聚苯胺(PANI)和氯化铁(II)的杂化复合材料制备的。一组使用联吡啶(BPy)作为额外的氮源和铁配体,提供了一种创新的方法。结果表明,热解温度和气氛的选择影响催化剂的性能。在热解过程中,氨的使用成为促进铁原子分散、增加表面积和孔隙率的关键参数。在0.5 M H2SO4(催化剂负载为0.6 mg cm−2)中,催化剂的半波电位为0.834 V,质量活性超过3 a g−1,在酸性电解质中具有很高的稳定性,是一种很有前途的非pgm结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

材料导报:能源(英文)
Renewable Energy, Sustainability and the Environment, Nanotechnology
CiteScore
13.00
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


