Greenness assessment profile of experimentally designed chromatographic method for the simultaneous analysis of ofloxacin and racecadotril in the presence of racecadotril impurities
IF 3.7
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
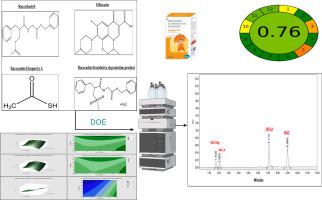
Experimental design beats the conventional one variable at a time (OVAT) to improve the chromatographic separations with lowest consumption of resources and maximum acceptable results for the separated peaks. Experimental design using face-centred composite design was utilized during method optimization to obtain the highest level of resolution with the fewest number of experimental trials for the determination of ofloxacin (OFLU) and racecadotril (RAC) in the presence of RAC impurities. The two independent variables were the pH of solvent A (0.10 % triethyl amine (pH 7 by orthophosphoric acid)) and its percentage in the mobile phase. RAC combined with OFLU is mainly used to treat diarrhoea in children. RAC has several impurities; among them is racecadotril impurity A (RAC-A) which was reported as a toxic impurity of RAC, additionally, RAC can be oxidized to racecadotril oxidative degradation product (RAC-Deg). Chromatographic separation was accomplished using Inertsil ODS column (150 mm, 4.6 mm, 5 μm) and isocratic elution using 0.10 % triethyl amine (pH 7 by orthophosphoric acid) (solvent A): methanol (solvent B) (20:80 v/v) as a mobile phase at a flow rate of 1.00 mL/min and UV detection at 240 nm. Sharp, well-resolved peaks for RAC-Deg, RAC-A, OFLU and RAC were obtained with retention times of 1.70, 2.06, 7.11, and 8.98 min, respectively. Method validation was carried out according to ICH guidelines and linearity was achieved in the ranges of 2.00–20.00 and 3.00–25.00 µg/mL for RAC and OFLU, respectively. The ecological impact of the presented technique was assessed utilizing the Analytical GREEnness Metric Approach and Software (AGREE) as an evaluation tool. The new approach shown high accuracy, selectivity, precision, and environmental friendliness in determining the mentioned drugs in pharmaceutical formulations. It is suitable for routine analysis of the drugs in quality control laboratories.
实验设计的同时分析氧氟沙星和消旋卡多曲杂质时消旋卡多曲的色谱方法的绿度评价曲线
实验设计优于传统的一次一变量(OVAT),以最低的资源消耗和最大的可接受的分离峰结果提高色谱分离。在方法优化过程中,采用面心复合设计,以最少的实验次数获得氧氟沙星(OFLU)和消旋卡多曲(RAC)在RAC杂质存在下的最高分辨率。两个自变量是溶剂A(0.10%三乙胺(正磷酸溶液pH为7))的pH及其在流动相中的百分比。RAC联合OFLU主要用于治疗儿童腹泻。RAC有几种杂质;其中外消旋卡多崔杂质A (raca)被报道为RAC的有毒杂质,RAC可被氧化为外消旋卡多崔氧化降解产物(RAC- deg)。色谱分离采用Inertsil ODS色谱柱(150 mm, 4.6 mm, 5 μm),流动相为0.10%三乙基胺(pH = 7,正磷酸)(溶剂A):甲醇(溶剂B) (20:80 v/v),流速为1.00 mL/min,紫外检测波长为240 nm。在保留时间分别为1.70 min、2.06 min、7.11 min和8.98 min时,获得了清晰、清晰的RAC- deg、RAC- a、OFLU和RAC峰。根据ICH指南进行方法验证,RAC和OFLU分别在2.00-20.00和3.00-25.00µg/mL范围内呈线性关系。利用分析绿色度量方法和软件(AGREE)作为评估工具,评估了所提出技术的生态影响。该方法测定制剂中上述药物具有较高的准确度、选择性、精密度和环境友好性。适用于质控实验室的药物常规分析。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


